Designing organic aquaculture systems: can we integrate microbial products and by-products?
Western consumers have, due to enhanced education and increased access to scientific and media services, become more sophisticated in their purchasing decisions.
In an age of bioterrorist threats, outbreaks of unusual zoonoses (e.g., transmissible bovine spongiform encephalitis, severe acute respiratory syndrome), increasing health concerns related to chemical contaminants (Hites et al., 2004) and the advent of genetically modified organisms, more attention than ever is being given to food quality and safety (Reid et al., 2004). This shift in consumer eating patterns has stimulated production of organic foods. As of the early 1980s, aquaculture represented the world’s fastest-growing food production sector.
However, since 1999, in many countries organic agriculture has supplanted aquaculture as the fastest-growing sector (FAO, 1999; El-Hage Scialabba and Hattam, 2002). The organic sector of agriculture is a US$28 billion industry, with more than 31 million hectares of farmland under organic management worldwide (IFOAM, 2006). Currently, there are organic standards for aquaculture in place for the European Union (EU), Canada, Japan, New Zealand, Australia, Chile, and Ecuador.
While there are currently no approved standards for organic aquaculture production in the United States, an interim final report from the Aquaculture Working Group was filed in late 2006 (USDA, 2006). This report summarized the adoption of standards that could lead to USDA Organic Certification of fish.
However, the adoption of these standards is still many years away. Industrial interest in organic aquaculture is based primarily upon the potential profitability of the organic sector (Craig and McLean, 2005). Although no official statistics are available with respect to organic aquaculture production, estimates suggest that in 2000 it did not exceed 5000 tons, which represents 0.01% of global aquaculture output (Bergleiter, 2001). This negligible production of certified aquaproduce underscores the difficulties inherent in achieving organic aquaculture standards.
The principal problem encountered relates to sourcing organic feed and nutrient resources (Tacon and Pruder, 2001). Based on current estimates of certified organic aquaculture production and anticipated growth of the industry, it has been predicted that organic aquaculture harvests will achieve 1.2 million tons by 2030 (El-Hage Scialabba and Hattam, 2002), still just 0.6% of predicted 2030 global aquaculture production. If such an increase is to be realized, however, new sources of certifiable feeds must be identified and investigated.
While the drive towards organic aquaculture production is real, given the small share of the total market it will represent, it is best if this movement is examined for the critical issues it is bringing to the forefront of the aquaculture industry as a whole—most notably, the movement towards true sustainability in terms of elimination of fish meal and fish oil products from aquafeeds.
Global protein production
While the need for fish meal reduction in aquafeed formulations has been discussed for over 30 years, reliance on this ‘gold standard’ of protein sources remains relatively high. Recent events have driven the aquaculture industry to finally incorporate many of the findings from these past studies.
Critical issues include the stagnant nature of the industrial fish supply, the increased cost of fuel oil, the impact of increased fuel costs on production efficiencies, the potential impact of increased quotas for ethanol and biodiesel production on the costs of more traditional feedstuffs (corn and soybean), and finally, and probably most importantly, the need for increased protein production to satisfy future protein needs of the global population.
This increased protein production in terms of animal production must have reliable and sustainable protein sources that are available for animal feed production. Clearly, we must consider alternative feedstuff protein sources as well as alternative production systems to drive this tremendous need for animal production. The aquaculture industry is certainly better positioned than the more traditional meat-producing industries due to high protein conversion efficiencies, higher consumption of seafood products in high demand areas (i.e., Asia), and finally, higher health status of most seafood products.
Single-cell protein sources
Single-cell proteins such as microalgae, bacteria, and yeast have been the least studied of all the alternative protein sources. Of these types of single-cell proteins, yeasts have been used the most frequently in aquafeed formulations (Oliva-Teles and Goncalves, 2001). Yeasts have a high nutritional value, because they are a rich source of proteins, B-complex vitamins, complex carbohydrates, such as glucans, and nucleotides (Oliva-Teles and Goncalves, 2001; Olvera-Novoa et al., 2002; Li and Gatlin, 2006). They are also low in phosphorus, which will lead to less water and environmental contamination than fish meal and other plant-based alternative protein sources that contain high levels of phosphorus (Cheng et al., 2004).
Yeasts represent a sustainable alternative protein source that is relatively inexpensive and easily produced on an industrial scale (Olvera-Novoa et al., 2002), and in most cases, can be certified organically. Utilization of yeasts as sources to replace fish meal in diets for numerous species has been investigated with varying levels of success. For instance, Rumsey et al. (1991) incorporated brewer’s dried yeast into diets for rainbow trout at 0, 25, 50, and 75%.
Results of this study showed that beyond 25% inclusion of brewer’s dried yeast, both growth and feed utilization declined significantly. Fish fed the 50 and 75% yeast diets would take the feed into their mouths and then expel it, suggesting that palatability was an issue. Perera et al. (1995) used a bacterial single-cell protein incorporated into diets for rainbow trout at 0, 25, 62.5, and 100%.
Results showed that final mean weights tended to decrease with increasing content of bacterial single-cell protein. An inclusion level of 25% was suggested for rainbow trout because at this level, no deleterious effects on feed consumption, absorption efficiency, or growth rate were noticed. In a study using sea bass juveniles, brewer’s yeast was used to replace up to 50% of the fish meal protein (Oliva-Teles and Goncalves, 2001).
Replacement of fish meal with up to 30% brewer’s yeast did not have any effects on growth rate or feed intake, and significantly improved feed conversion. Olvera-Novoa et al. (2002) used torula yeast as a dietary protein source for tilapia fry and replaced meat meal by up to 45% of dietary protein. Highest growth responses were observed for fish fed the 30% yeast protein diet. Feed acceptance and survival were not affected by dietary yeast content.
Another benefit of the use of yeasts in diets for fish is their nucleotide content. Although nucleotides are not required nutrients since they can be synthesized endogenously, under conditions of rapid growth dietary nucleotides may be beneficial, since rapid growth limits the de novo synthesis of these molecules from their amino acid precursors (Carver, 1999).
Studies involving tilapia larvae (Ramadan and Atef, 1991), juvenile rainbow trout (Adamek et al., 1996), and Atlantic salmon (Burrells et al., 2001) have shown improvements in weight gain with the inclusion of dietary nucleotides.
Alternative protein research at Virginia Tech
While the above studies involving single-cell protein utilization in aquafeeds demonstrated detrimental impacts on production characteristics at inclusion rates above 30%, research at the Virginia Tech Aquaculture Center (VTAC) has produced several successes at replacement rates as high as 75% of the fish meal protein. Utilizing NuPro® (Alltech Inc.) not as a feed additive, but as a true alternative protein source, research conducted at VTAC has completely replaced both soybean meal and fish meal in diets as the protein source in tilapia diets without diminishing the extent of weight gain (Craig and McLean, 2005).
In fact, tilapia fed diets containing NuPro® had more growth (319-458%) than those fed the commercial diet of fish meal and soybean meal (277%), except for the 100% NuPro® diet in which no difference in weight gain was observed.
In numerous trials conducted with cobia, a high level marine carnivore, NuPro® was used effectively as a fish meal replacement at levels of 40% without detrimental impacts on production characteristics (Lunger et al., 2006; 2007). Higher levels of 50 and 75% replacement of fish meal were successful when supplemental amino acids were included in the formulation.
The most surprising aspect of this research was the positive impact of taurine, an essential amino acid for true carnivores. At NuPro® inclusion rates of 50 and 75%, weight gain actually exceeded that of controls when taurine was incorporated at 0.5% of dry diet. Taurine could have acted as a feed attractant (Martinez et al., 2004), thereby negating potential palatability issues observed by other researchers when incorporating high levels of yeast proteins (see: Craig and McLean, 2006). More intriguing from a sustainability standpoint is the potential that taurine becomes conditionally indispensable when carnivorous fish are fed high levels of plant-based protein sources.
Finally, research with NuPro® at VTAC, in collaboration with the Organic Aquaculture Institute (OAI), has resulted in the first commercial production of Litopenaeus vannamei with aquafeeds containing no fish products and utilizing novel production methods for organically certifiable marine shrimp.
The pond as a ‘rumen’: feeding the microbes
The microbial community in seawater is a diverse and multifaceted niche that is often overlooked in terms of its tremendous impact on primary productivity and food web structures as a whole (Azam, 1998). Estimates are that almost half of the carbon fixed in primary production flows through the bacterial/protozoa pathway.
Shrimp ponds are no exception in terms of the microbial food web and its components, although the inputs into the shrimp pond system are more continuous and low in the carbon:nitrogen (C:N) ratio due to the high protein feeds often utilized by shrimp producers. It is obvious from the field trials at the OAI that the microbial food web can be exploited at low stocking densities (<25/m2) to produce high quality shrimp at much reduced costs, i.e., feed inputs, but this type of production mentality is a paradigm shift in terms of traditional shrimp farming practices.
While the recent trend in shrimp production has been toward super-intensification of shrimp ponds as well as zero-exchange technologies that exploit the microbial community (Wasielesky, Jr. et al., 2006), this intensification cannot overcome the associated increased feed, energy and labor costs of these systems when compared with production in developing countries.
This new paradigm for shrimp farming achieves organic certification criteria, incorporates lower stocking densities, exploitation and natural manipulation of the microbial food web, total elimination of fishery products from feeds, enhanced environmental and animal welfare and a higher value product. Very possibly it could represent the only method through which US shrimp farmers will be able to maintain production and viability as an industry.
It has been recognized for almost a quarter century that the natural productivity of shrimp ponds provides a supplemental source of nutrients for growing shrimp. For example studies have shown that between 53 and 77% of shrimp growth in a pond is due to grazing on pond biota (Anderson et al., 1987).
As shrimp are slow consumers of feed, many nutrients are released into the pond environment (Decamp et al., 2002) and thus become available for bacteria through particulate organic matter (Azam et al., 2002). Of course, stocking densities are a major influence on microbial utilization in grow-out production ponds, but in essence, shrimp producers are feeding the microbes, which in turn are feeding the animal—a variation of the ruminant model.
While this analogy may appear somewhat simplistic, a comparison between a shrimp pond and a rumen is actually a good one. Given that shrimp feeds are often the most costly of the aquafeeds, this is clearly not the most economical manner in which to culture marine shrimp. If shrimp aquafeeds are mainly feeding the microbes, then at the very least, less costly formulations are in order, especially under low stocking densities mandated by most organic certifiers. Better is to fully exploit the microbial food web to further reduce feeding costs and increase the profit margin for the producer.
Fertilizers have traditionally been applied to increase the production of prey items in aquaculture ponds (Boyd, 1990), but these have fallen short in terms of actually manipulating the microbial food web to optimize its management for shrimp production.
Adjustments in the C:N ratio must be made to fully manipulate the nutrient flux pathways so that bacterial growth is stimulated, bacterial biomass is transferred through the trophic levels and a carbon-rich media is added to encourage bacterial growth on a particle that is within the optimum size range for penaeid shrimp (Azam et al., 2002; Burford et al., 2003; Hargreaves, 2006; Kuhn et al., 2007). Bearing these issues in mind, commercial-scale trials at the OAI have led to novel feeding strategies that attempt to more fully exploit and manage the microbial food web under organic production regimes.
A case study with shrimp and NuPro®
In field trials during the 2005 and 2006 growing seasons, NuPro® was utilized as a replacement protein for fish meal in shrimp aquafeeds. These commercial-scale production runs were conducted under culture conditions that had previously been certified as organic. In these trials at the OAI facilities in Imperial, Texas, shrimp were stocked at low stocking densities (10-12 animals/m2) and fed either a common commercial shrimp aquafeed, or one that replaced the fish meal component with NuPro®.
In addition, the ponds were fertilized with organic compost, derived from dairy waste at a rate of 45-50 kg/pond/ either bimonthly (2005) or daily (2006), to manipulate pond C:N ratios and thus maintain the microbial community at high levels for potential grazing by the shrimp (Moss, 2002). Additionally, the compost served as a substrate for further bacterial growth on which the shrimp could graze.
In 2005 trials, diets were formulated to provide 35% crude protein (CP), either from a standard commercial diet, or in a diet in which NuPro® replaced all the fish meal on an isonitrogenous basis. In all ponds, the NuPro®-fed shrimp were larger and had smaller individual variability when compared with shrimp from the control ponds (Figure 1).
Survival in all ponds averaged 80% compared with a mean from 24 other shrimp farms in Texas of only 50%. Feed conversion ratios (g fed/g gained) were 0.5 for both the control and the NuPro®-fed shrimp (McLean et al., 2006). That is, it took 0.5 kg of feed to produce 1 kg of shrimp! Typical shrimp FCR range from 1.5-2.0 when utilizing traditional culture practices. Clearly, the microbial community contributed significantly to the nutritional well-being of the culture system and the addition of compost maintained optimal C:N ratios for further microbial community enhancement.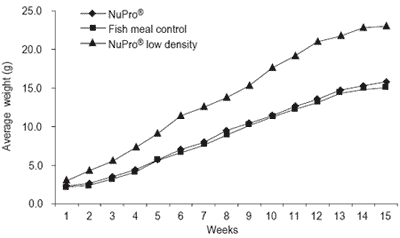
Figure 1. Weekly average weights of shrimp fed either a commercial shrimp aquafeed or one that replaced 100% of the fish meal with NuPro® on an isonitrogenous basis. The low density pond was stocked at 50% of the other ponds and fed the same NuPro®-based diet.
All diets provided 30% crude protein on an as-fed basis.
In 2006, NuPro® again replaced all the fish meal from a commercial shrimp formulation, but the protein levels were dropped to 30% CP. A standard commercial shrimp aquafeed containing 30% CP diet served as the control. Shrimp were stocked at 10-12 animals/ m2.
There were no differences in weight gain in shrimp fed either diet with increases from initial weight ranging from 550 to 610% and specific growth rates of approximately 2% body weight per day (Figure 2). Final harvest weight was 15.8 g/shrimp for the NuPro®-fed ponds compared with 15.1 g/shrimp for the control ponds. In a parallel study, one pond was stocked at lower densities (5 shrimp/m2) but fed using the identical protocols and NuPro® diet.
This study was undertaken to assess the impact of stocking densities and lower protein feeds on animal growth potential. Shrimp in this pond reached an average harvest weight of 23.1 g. Two major points are to be made on this observation.
First, and most importantly, marine shrimp can be grown up to a large size (23+ g) in a single growing season, utilizing a diet without fish meal and at lower than normal levels of protein, when the pond is supplemented with compost. Moreover, these studies at the OAI have been successfully completed at a commercial scale over two consecutive production cycles demonstrating categorically that this new paradigm for shrimp production has significant merit.
Clearly the application of organic compost was a success in terms of providing the pond environment with a higher C:N ratio as well as a substrate for bacterial growth within the optimal forage size for marine shrimp. However, these trials certainly need further refinement, and the results generated are likely to be regionspecific due to variations in microbial dynamics. Nevertheless the basic premise has been validated on a commercial scale and future trials will place greater emphasis upon nutrient flux pathways from the microbial community.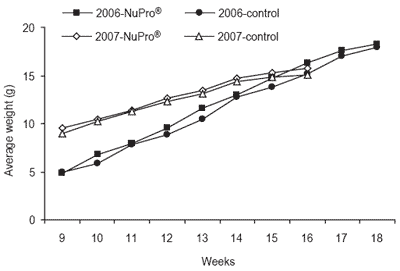
Figure 2. Growth performance of marine shrimp over two consecutive production years. No differences were apparent between animals fed a traditional fish meal-based diet and those in which NuPro® replaced 100% of the fish meal.
Implications
The use of organic compost, coupled with stocking rates that can be certified under organic production guidelines, points to a novel, alternative culture system and methodology that could serve to save the US shrimp industry.
Presently, shrimp producers in the US simply cannot compete with imports due to the drastically reduced production costs in developing countries, where a majority of farmed shrimp originates. This scenario is unlikely to change in the near future, barring a ban on imports by the US government, something highly unlikely.
However, by utilizing this organic production system, in conjunction with novel feeds such as NuPro®, while total yields will be lower due to stocking densities, production costs will be dramatically reduced.
For example, utilizing the data from our field trials, a producer could save over 70% on feed costs by effectively utilizing the microbial food web, and thus the pond as a rumen. Add to feed savings the potential for higher margins for product produced under organic guidelines and the profit for the farmer is truly maximized, while helping the environment in terms of utilization of a ‘waste’ stream from another industry (dairy waste).
Additionally, the system becomes sustainable due to the elimination of fish products from the feeds, relying instead upon a unique sustainable, organically certifiable protein source (NuPro®). Concomitantly the emphasis being placed on biofuel production and in turn the severe pressure this will place on other sources of animal feedstuffs in the coming years, new and innovative feeding strategies and ingredients will be needed to change the future of animal production.
References
Authors: STEPHEN R. CRAIG1,2 and EWEN McLEAN2Adamek, Z., J. Hamackova, J. Kouril, R. Vachta and I. Stibranyiova. 1996. Effect of Ascogen probiotics supplementation on farming success in rainbow trout (Oncorhynchus mykiss) and wels (Silurus glais) under conditions of intensive culture. Krmiva (Zagreb) 38:11-20.
Anderson, R.K., P.L. Parker and A. Lawrence. 1987. A 13C/12C tracer study of the utilization of presented feed by a commercially important Penaeus vannamei in a pond growout system. J. World Aqua. Soc. 18:148-155.
Azam, F. 1998. Microbial control of oceanic carbon flux: the plot thickens. Science 280:694-696.
Azam, F., S. Haskell and F. Rohwer. 2002. The microbial loop in aquaculture. In: Microbial Approaches to Aquatic Nutrition within Environmentally Sound Aquaculture Production Systems. (C.S. Lee and P. O’Bryen, eds.). World Aquaculture Society, Baton Rouge, LA. pp. 87-95.
Bergleiter, S. 2001. Organic products as high quality niche products: background and prospects for organic freshwater aquaculture in Europe. Paper presented at the ad hoc EIFAC/EU Working Party on Market Perspectives for European Freshwater Aquaculture, 12-14 May 2001, Brussels, Belgium.
Boyd, C.E. 1990. Water Quality in Ponds for Aquaculture. Birmingham Publishing Company, Birmingham, AL.
Burford, M.A., P.J. Thompson, R.P. McIntosh, R.H. Bauman and D.C. Pearson. 2003. Nutrient and microbial dynamics in high intensity, zero-exchange shrimp ponds in Belize. Aquaculture 219:393–411.
Burrells, C., P.D. Williams, P.J. Southgate and S.L. Wadsworth. 2001. Dietary nucleotides: a novel supplement in fish feeds 2. Effects on vaccination, salt water transfer, growth rates and physiology of Atlantic salmon (Salmo salar L.). Aquaculture 199:171-184.
Carver, J.D. 1999. Dietary nucleotides: effects on the immune and gastrointestinal systems. Acta Paediatrics 88:83-88.
Cheng, Z.J., R.W. Hardy and N.J. Huige. 2004. Apparent digestibility coefficients of nutrients in brewer’s and rendered animal by-products for rainbow trout (Oncorhynchus mykiss (Walbaum)). Aquaculture Research 35:1-9.
Craig, S.R. and E. McLean. 2005. The organic aquaculture movement: a role for NuPro® as an alternative protein source. In: Nutritional Biotechnology in the Food and Feed Industries: Proceedings of Alltech 21st Annual Symposium (T. P. Lyons and K.A. Jacques, eds). Nottingham University Press, UK.
Craig, S.R. and E. McLean, 2006. Sustainable aquaculture of cobia: A case study with organically certified alternate proteins. In: Nutritional Biotechnology in the Food and Feed Industries: Proceedings of Alltech’s 22nd Annual Symposium (T.P. Lyons, K.A. Jacques and J.M. Hower, eds). Nottingham University Press, UK.
Decamp, O., L. Conquest, I. Forster and A.G.J. Tacon, 2002. The nutrition and feeding of marine shrimp within zero-water exchange aquaculture production systems: role of eukaryotic microorganisms. In: Microbial Approaches to Aquatic Nutrition within Environmentally Sound Aquaculture Production Systems. (C-S Lee and P. O’Bryen, eds). World Aquaculture Society, Baton Rouge, LA. pp. 79-85.
El-Hage Scialabba, N. and C. Hattam (eds.) 2002. Organic Agriculture, Environment and Food Security. Environment and Natural Resources Service Sustainable Development Department, FAO, Rome, Italy.
FAO, 1999. Committee on Agriculture, 15th Session. Rome, 25-29 January 1999. FAO, Rome, Italy.
Hargreaves, J.A. 2006. Photosynthetic suspended-growth systems in aquaculture. Aquaculture Engineering 34:344-363.
Hites, R.A., J.A. Foran, D.O. Carpenter, M.C. Hamilton, B.A. Knuth and S.J. Schwager, 2004. Global assessment of organic contaminants in farmed salmon. Science 303:226- 229.
The International Federation of Organic Agriculture Movements (IFOAM), 2006. The world of organic agriculture: More than 31 million hectares worldwide. http:// www.ifoam.org/press/press/Statistics_2006.html.
Kuhn, D.D., G.D. Boardman, S.R. Craig, G.J. Flick Jr. and E. McLean. 2007. Use of microbial flocs generated from tilapia effluent as a nutritional supplement for Litopenaeus vannamei in RAS. J. World Aqua. Soc. (Accepted).
Li, P. and D.M. Gatlin. 2006. Nucleotide nutrition in fish: Current knowledge and future applications. Aquaculture 251:141-152.
Lunger, A.N., E. McLean and S.R. Craig. 2006. Replacement of fish meal in cobia (Rachycentron canadum) diets using an organically certified protein. Aquaculture 257:393-399.
Lunger, A.N., E. McLean and S.R. Craig. 2007. The effects of organic protein supplementation upon growth, feed conversion and texture quality parameters in juvenile cobia (Rachycentron canadum). Aquaculture, in press.
Martinez, J.B., S. Chatzifotis, P. Divanach and T. Takeuchi. 2004. Effect of dietary taurine supplementation on growth performance and feed selection of sea bass Dicentrarchus labrax fry fed with demand-feeders. Fisheries Sci. 70:74-79.
McLean, E., B. Reid, D. Fegan, D. Kuhn and S.R. Craig. 2006. Total replacement of fish meal with an organically certified yeast-based protein in Pacific White shrimp (Litopenaeus vannamei) diets: laboratory and field-based trials. Ribarstvo. 64:47-68.
Moss, S.M. 2002. Dietary importance of microbes and detritus in Penaeid shrimp aquaculture. In: Microbial approaches to aquatic nutrition within environmentally sound aquaculture production systems. (C.S. Lee and P. O’Bryen, eds), World Aquaculture Society, Baton Rouge, USA, pp. 1–18.
Oliva-Teles, A. and P. Goncalves. 2001. Partial replacement of fish meal by brewer’s yeast (Saccaromyces cerevisae) in diets for sea bass (Dicentrarchus labrax) juveniles. Aqua. 202:269-278.
Olvera-Novoa, M.A., C.A. Martinez-Palacios and L. Olivera-Castillo. 2002. Utilization of torula yeast (Candida utilis) as a protein source in diets for tilapia (Oreochromis mossambicus Peters) fry. Aqua. Nutr. 8:257-264.
Perera, W.M.K., C.G. Carter and D.F. Houlihan. 1995. Feed consumption, growth, and growth efficiency of rainbow trout (Oncorhynchus mykiss (Walbaum)) fed on diets containing a bacterial single-cell protein. Br. J. Nutr. 73:591-603.
Ramadan, A. and M. Atef. 1991. Effect of the biogenic performance enhancer (Ascogen ‘S’) on growth rate of tilapia fish. Acta Vet. Scand. 87:S304-S306.
Reid, B., E. McLean and S.R. Craig. 2004. Performance characteristics of shrimp (Litopenaeus vannamei) fed a certified organic feed versus an investigational organic aquafeed. In: Proceedings of the 5th International Conference on Recirculating Aquaculture (G.J. Flick, A. Correa and T. Rakestraw, eds), July 24-27th 2004, Roanoke, Virginia, USA, pp. 539-542.
Rumsey, G.L., J.E. Kinsells, K.J. Shetty and S.G. Hughes. 1991. Effects of high dietary concentrations of brewer’s dried yeast on growth performance and liver uricase in rainbow trout (Oncorhynchus mykiss). Anim. Feed Sci. and Technol. 33:177-183.
Tacon, A.G.J. and G.D. Pruder. 2001. Opportunities and challenges to organic certification of aquatic animal feed. In: Final report of the National Organic Aquaculture Workshop (D.J. Brister and A. Kapuscinski, eds), June 23-24, 2000. St. Paul, USA, Institute for Social Economic and Ecological Sustainability, University of Minnesota. pp. 26-42.
USDA. 2006. Interim final report of the National Organic Working Group, Winter 2006. http://www.ams.usda.gov/nop/TaskForces/AATFInterimFinalReport.pdf.
Wasielesky, Jr., W., H. Atwood, A. Stokes, and C.L. Browdy. 2006. Effect of natural production in a zero-exchange suspended microbial floc based super intensive culture system for white shrimp, Litopenaeus vannamei. Aquaculture 258:396-403.
1 Virginia/Maryland Regional College of Veterinary Medicine
2 Department of Fisheries and Wildlife Sciences, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, USA




.jpg&w=3840&q=75)