INTRODUCTION
The medical plants are not only used in the ancient nations as folkloric drugs but also in the new world as a return to the nature to avoid the negative side effects of the chemical (pharmaceutical or synthetic) drugs. Many attempts were cariied out to utilize from these plants as appratives or growth promters (El-Saidy and Gaber, 1997; Gaber, 2000; Abd Elmonem et al., 2002 and Abd El-Hakim, 2008) or whether to substitute one of the conventional feed stuffs in a diet or for their attractive or immunostimulatory effects for their constituents'effects (Tawfik et al., 2005). Mint (Mentha in Italic) is a plant shrub genus which contains 13 – 18 species (their origin from europ and Asia), among these is the field ment (Mentha arvensis), it is a permanent green plant which reaches ca. 50 cm length, and produces ca. 25 – 30 kg oil and one ton dried leafes / feddan / year. According to the American Ministry of Agriculture, each 100 g of mint contain 70 kcal., 0.94 % fats, 0.24 % saturated fats, 14.89 % carbohydrates, 8 % fibers, and 3.75 % proteins. It is penifet for curing billary disruptions, intestinal coli and stomach gases, besides its effect as tonic. Its inclusion of mantol treats spasms, hepatits, and enflamations. It is used in producing drugs, cosmetics, sweetness, and drinks. Green (Japanise) tea (Camellia sinensis) is originated from China, India and Silan and introduced to Japan 800 year B.C. It can reduce blood viscosity and cholesterol level (but increase the high density lipo-protein) and act as antioxidant, anti-carcinogen, immuno-stimulant, intestinal motility stimulator, and anti-toxins (anti-food poisoning). It acts against poisoning bacteria and increases beneficial bacteria. Yet, it reduces the absorption of calcium and iron (Anon., 2010). German matricaria(Matricaria chamomilla) is an annual shrub (5 – 15 cm length), with aromatic flavour, its origin from Arab world, Europ, and Turki. Its dried flowers contain 1.5 % volatile oil. This oil contains many effective substances as alpha bisabolol, bisabolol oxide A, bisabolol oxide B, β- trans – farnesene, chamaxulene, spathulenol, umbeliferone, herniarin, and flavone glycosides (as apigenin, crysoeriol, luteoline, rutin, quercetin, iserhamntin), as well as hydroxycoumarins (10 %). Azulene is the most important component in the oil; it is responsible for the curing effect of Matricaria chamomilla, since it contains unsaturated fatty acids. Matricaria chamomilla contais tannins, but it is helpsamfull as apperative, anti-fungal, hepatic tonic, gas spiller, anti-colic, sedative, anti-diarhea, immuno-stimulant, and is used in cosmetic soap (Anon., 2013). Marjoram (Mvajorana hortensis or Origanum majoranum) is originated from Medditerranean sea basen, it is a permanent green shrub of 50 – 60 cm length, 25 – 30 kg aromatic oil and 2 – 2.5 ton dried leaves / feddan / year. The Egyptian marjoram is a world wide good one, Egypt exportes yearly ca. 2000 – 2500 ton of dried marjoram leaves. It is used as food spices, and is used in pharmaceuiticl products for its anti-microbial, anti colic, anti spasm effects; as well as in parfeums and cosmetics production. It is usefull for pituitary gland, liver, colon, and pancreas (Ibrahim, 2013). Ginger root (Zingiber officinale) contains volatile oil (, China produced 25 % of the total world production of ginger year 2005. It is used as tonic, food spices, and hot drinks. It is also benefit for treating liver and kidney disorders, gout, stomach gases, mal-dygestion, colic, vascular disorders, and sexual dysfunction. But it may interact with absorption of iron, fat soluble vitamins and some antibiotics. The Indian ginger is the most usable. Its raw roots are potassium and phosphorus rich (USDA, 2008). Cinnamum(Cinnamomum zeilanicum Nees or Cinnamomum zeylanicum or Cinnamomum verum) was known for ancient Egyptian. It is a permanent green tree of 10 – 40 m length, originated from south East Asia and South America. Its park contains ca. 40 % volatile oils and is rich in magnesium, iron and calcium and contains cinnamaldehyde as antioxidant. It reduces blood cholesterol; so, it is useful for heart and used in treating hernia, inflammations, fatness, diabetic, as well as white candida (Alsaghir, 2010). So, the present work aimed to evaluate some medicinal herbs as feed additives to common carp fry.
MATERIALS AND METHODS
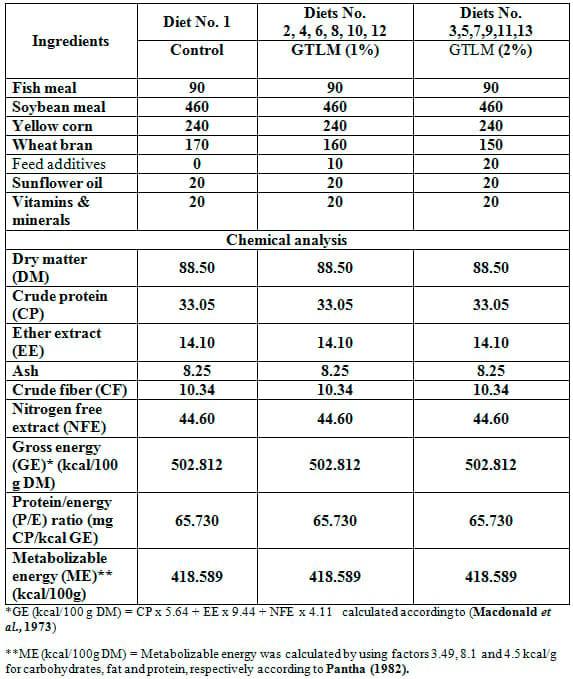
An indoor feeding experiment was conducted with common carp (Syprinus carpio) fry (0.07 – 0.09 g) for 12 weeks to evaluate the dietary inclusion of 1: Mentha arvensis leaves meal (MALM) 2: Camellia sinesis leaves meal (CSLM), 3: Marticaria chmomilla meal (MCM), 4: Origanum majorana leaves meal (MOLM), 5: Zingiber officinale meal (ZOM), and 6: Cinnamum zeylanicum meal (CZM) at 3 levels each, being 0, 1, and 2 % of the diet. A group of common carp fry was obtained from the Fish Nutrition Lab., Baltim Research Station for Aquatic Resources, National Institute of Oceanography and Fisheries, Egypt and transported to the wet lab. Fish were maintained in tanks for 2 weeks before the beginning of the experiment for acclimatization purpose. The fish were fed during the acclimatization period on the basal diet (30 % crude protein) at a rate of 20 % of the body weight daily, at 2 times daily. The experimental treatments were tested at three tanks (replicates) for each. Fish were stoked at a density of 5 fish / tank. Ground dried leavs (Mentha arvensis, Camellia sinesis, Marticaria chmomilla, and Origanum majorana), roots (Zingiber officinale), and wood (Cinnamum zeylanicum) were added to diets. All feedstuffs used in the experimental diets were purchased from the local market. Composition and chemical analysis of the basal and experimental diets are presented in Table 1 which showed that all the experimental diets were iso-caloric and iso-nitrogenous. The composition of the vitamins and minerals mixture is presented in Table 2. The evaluation included growth performance, carcass composition and feed utilization. The experimental system consisted of 39 plastic tanks (each of 16 liter water), each tank was continuously supplied with a compressed air from an electric compressor (Shenzehe Company BS410). Dechlorinated tap water was used to change one third of the water in each tank every day. Water was aerated for about 24 hours to remove chlorine before be used.
Table 1: Composition (%) and chemical analysis (% dry matter bases) of the experimental diets.
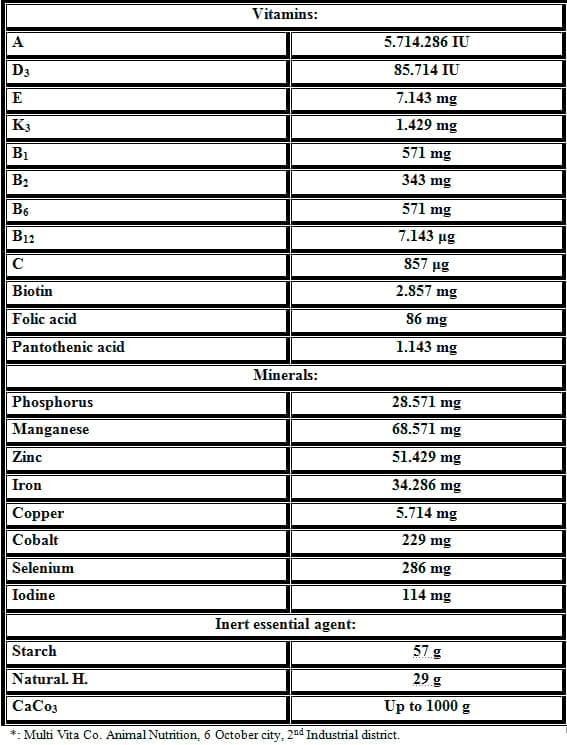
Table 2: Composition of the vitamins and minerals mixture*(calculated for each kg of the mixture) in the diets.
During the experimental period, the fish were fed the experimental diets at a rate of 20 % of the live body weight daily. The diet was introduced twice daily, at 8 a.m. and 2 p.m. The amount of feed was adjusted weekly based on the actual body weight changes. Light was controlled by a timer to provide a 14 h light: 10 h dark as a daily photoperiod. Samples of water from each aquarium were taken to determine water temperature, pH value, and dissolved oxygen concentrations according to Abdelhamid (1996). Water temperature in degree centigrade was recorded every day by using a thermometer. The pH value of water was measured daily using an electric digital pH meter (Jenway Ltd, model 350-pH meter). Dissolved oxygen concentration was determined weekly using an oxygen meter model (d-5509). Determinations of proximate composition ofdiets and fish body at the start and at the end of the experiment for different groups were carried out according to the methods of A.OA.C. (1990). At the end of the experiment, three fish were derived from each tank for drying at 60ºC for 48 hours and then milled through electrical mill and kept at 4oC until analysis. The growth performance and feed utilization parameters were calculated according to the following equations:
Average weight gain (AWG, g/fish) = Average final weight (g) - Average initial weight (g).
Average daily gain (ADG,mg/fish) = [Average final weight (g)-Average initial weight (g)] 1000 / Time (days).
Survival rate (SR %) = Total number of fish at the end of the experiment × 100 / total number of fish at the start of the experiment.
Relative growth rate (RGR) = Average weight gain (g) / Average initial weight (g).
Specific growth rate (SGR, % / day) = 100 [ln wt1- ln wto/T].
Where: ln: Natural log. Wto: Initial weight (g), Wt1: Final weight (g), and T: Time in days.
Feed conversion ratio (FCR) = Total feed consumption (g) / Weight gain (g).
Protein efficiency ratio (PER) = Body weight gain (g) / protein intake (g).
Protein productive value (PPV %) = 100 [Retained protein (g) / protein intake (g)].
Energy retention (ER %) = 100 [Retained energy (Kcal) / Energy intake (Kcal)].
The data were statistically analyzed using General Linear Models (GLM) procedure adapted by SAS (1996) for user's guide. Means were separated using Duncan's multiple range test (Duncan, 1955).
RESULTS
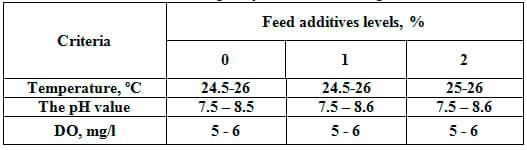
Water quality parameters measured (temperature, pH and dissolved oxygen) did not differ among treatments (Table 3). Values of the measured criteria were within the normal-suitable ranges for rearing freshwater fish (being 24– 26 ºC, 7.5 – 8.5, and 5 – 6 mg/l, respectively) according to Abdelhamid (2009).
Table 3: Means of some water quality criteria in the experimental fish tanks
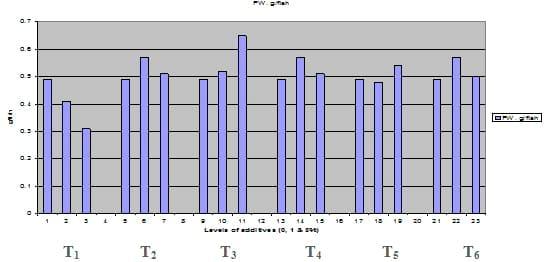
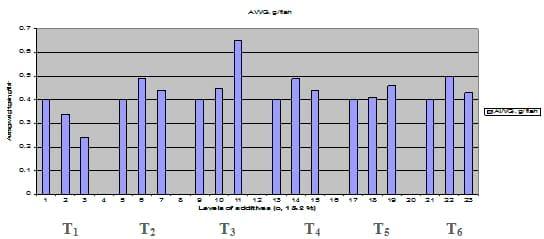
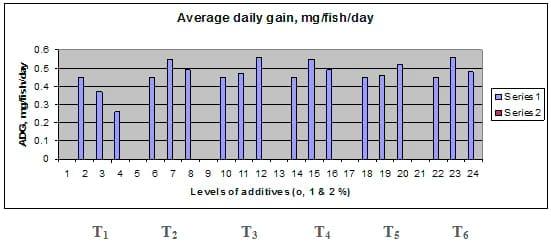
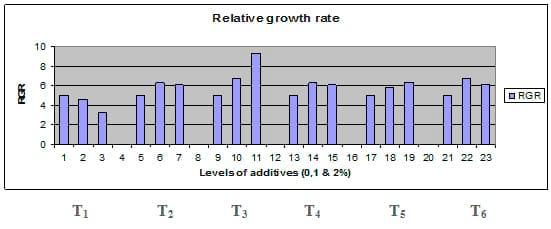
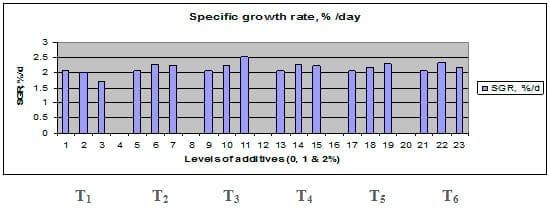
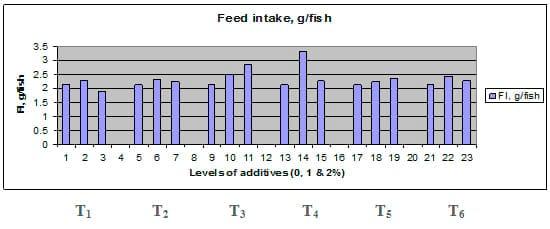
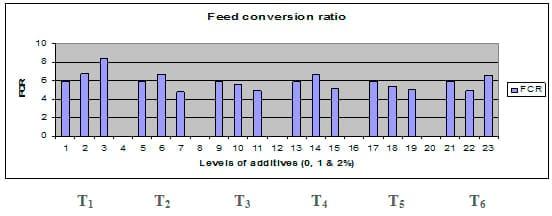
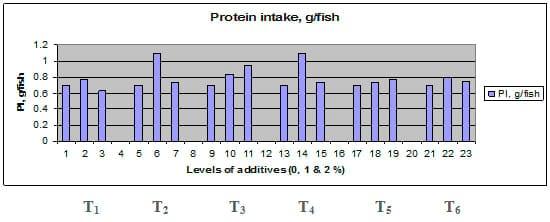
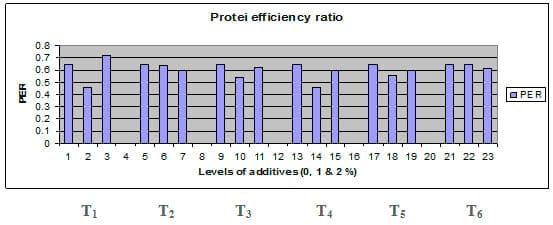
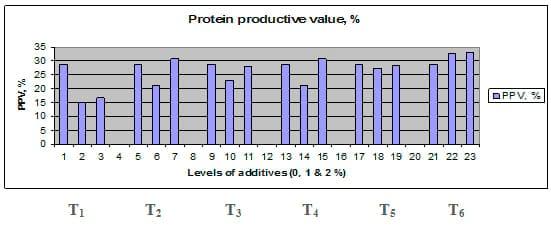
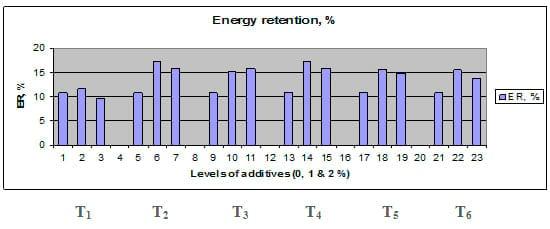
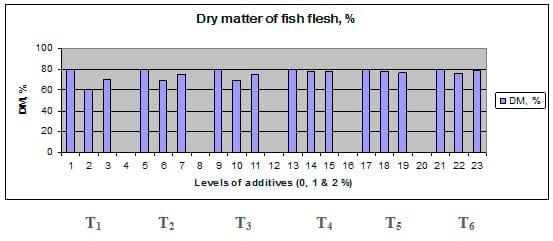
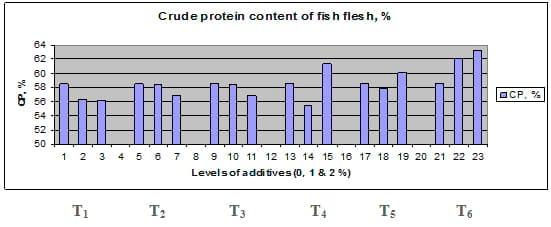
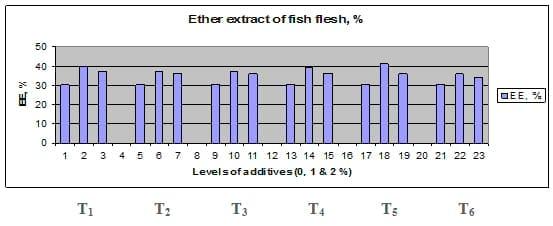
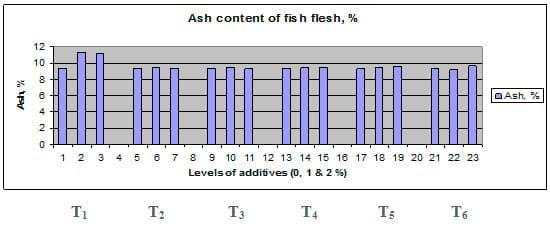
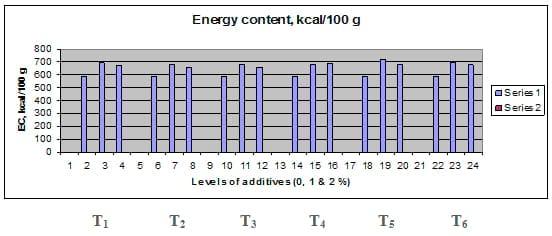
Figures 1 – 6 illustrate means of growth performance criteria measured including FW, AWG, ADG, RGR and SGR besides SR by common carp fry fed 6 feed additives at 3 levels each for 12 weeks. All growth performance parameters [final bodyweight (FBW), average weight gain (AWG) and average daily gain ADG), relative growth rate (RGR) and specific growth rate (SGR)] were significantly (P≤0.05) better on control diet than on the 1st treatment (menthe), particularly at the highest level (2 %). Yet, there were no significant (P≥0.05) differences among treatments for survival rates (SR). The same trend was noticed concerning feed and nutrients utilization (Figs. 7 – 12), since feed intake (FI), feed utilization (FU) efficiency, protein intake (PI), protein productive value (PPV) and energy retention (ER)were more better (P≤0.05) on the control diet than on the treatment No.1 (menthe), particularly at the highest level (2 %). Yet, protein efficiency ratio was the lowest (P≤0.05) at 1 % menthe-diet comparing with both other diets. The control diet reflected the highest dry matter (DM) and crude protein (CP) and the lowest (P≤0.05) ether extract (EE), and ash percentages and lowest (P≤0.05) carcass energy content (EC) in fish body, in comparison with both other diets (Figs. 13 – 17). Treatment No. 2 (green tea) improved significantly (P≤0.05) FBW, AWG, ADG, RGR and SGR at its both levels (1 and 2 %) comparing with control (0 % green tea), but did not influence (P≥0.05) SR. Increases (P≤0.05) were noticed in FI, PI and ER at 1 and 2 % green tea diets, but improvements were found in FCR and PPV at 2 % green tea diet comparing with both other diets, the opposite was true for PER, which was the lowest (P≤0.05) at 2 % green tea diet. Best (P≤0.05) DM, EE, ash and EC were given by the control diet, but highest (P≤0.05) CP was obtained in body of fish fed 1 % green tea diet. Marticaria (treatment No.3) at its both levels (1 and 2 %) increased significantly (P≤0.05) each of FBW, AWG, ADG, RGR, SGR, FI, PI, and ER, but did not influence significantly (P≥0.05) SR; yet, improved FCR (at 2 % marticaria) and worst PER and PPV (particularly at 1 % marticaria, P≤0.05) comparing with the control diet. Better (P≤0.05) DM, EE, ash and EC were given in fish body fed the control diet comparing with marticaria diets; yet, marticaria diets offered significantly (P≤0.05) higher CP, particularly at 1 % level. Origanum is the 4th treatment, its both levels significantly (P≤0.05) increased FBW, AWG, ADG, RGR, SGR, FI, PI and ER, but did not significantly (P≥0.05) affect SR. Yet, its 2 % level improved significantly (P≤0.05) FCR and PPV. However, it's both levels reduced PER, particularly at 1 % level (P≤0.05). It's both levels increased (P≤0.05) each of DM, CP, EE, ash and EC. Zingiber (treatment No. 5) significantly (P≤0.05) increased each of FBW (particularly the 2nd level (2 %)), AWG, ADG, RGR and SGR, FI, PI and ER although did not significantly (P≥0.05) influence SR. Although zingiber significantly (P≤0.05) worst each of FCR, PER and PPV (particularly the 1st level (1 %)), it significantly (P≤0.05) increased percentages of the fish body chemical composition (DM, EE, ash and EC). Ginger (treatment No. 6) at it's both levels increased each of FBW, AWG, ADG, RGR, SGR, FI, feed efficiency (at 1% only), PI, PPV and ER without any effect on SR, but PER was reduced at 2 %. Proximate analysis (CP, EE, ash and EC) of fish body increased significantly (P≤0.05) by feeding the ginger diets to carp fish.
Fig. 1: Final bodyweight (FW, g / fish) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 2: Average bodyweight gain (AWG, g / fish) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 3: Average daily gain (ADG, mg / fish / day) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 4: Relative growth rate (RGR) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 5: Specific growth rate (SGR) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 6: Survival rate (SR) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 7: Feed intake (FI, g / fish) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 8: Feed conversion ratio (FCR) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 9: Protein intake (PI, g / fish) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 10: Protein efficiency ratio (PER) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 11: Protein productive value (PPV) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 12: Energy retention (ER) of common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each.
Fig. 13: Dry matter (DM) percentage of carcass from common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each for 12 weeks.
Fig. 14: Crude protein (CP) percentage of carcass from common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each for 12 weeks.
Fig. 15: Ether extract (EE) percentage of carcass from common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each for 12 weeks.
Fig. 16: Ash percentage of carcass from common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each for 12 weeks.
Fig. 17: Energy content (EC, kcal./100 g) of carcass from common carp fry fed 6 feed additives at 3 levels (0, 1 and 3%) each for 12 weeks.
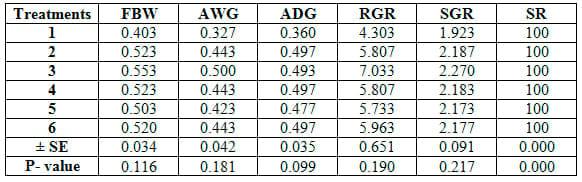
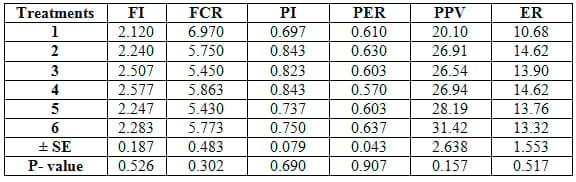
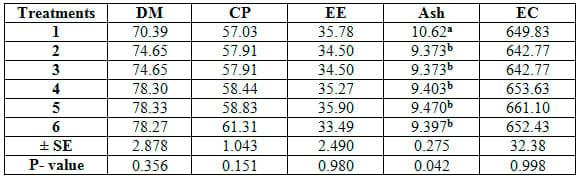
The comparison among different feed additives used herein reveald no significant (P≥0.05) differences among six treatments in growth performance parameters (Table 4) or feed and nutrients utilization criteria (Table 5). Yet, concerning proximate analysis of fish carcass, ash content only was significantly (P≤0.05) higher in T1 than the other treatments (Table 6).
Table 4: Growth performance parameters of common carp fry fed 6 feed additives for 12 weeks
Table 5: Feed efficiency parameters of common carp fry fed 6 feed additives for 12 weeks
Table 6: Carcass composition of common carp fry fed 6 feed additives for 12 weeks
DISCUSSION
Similar results concerning the positive efffects on growth performance, feed and nutrients utilization and chemical composition were obtained by El-Saidy and Gaber (1997) and Aly et al. (2008) when fed Nile tilapia the garlic meal supplement, Gaber (2000) by feeding Nile tilapia on clove (Eugenia caryophyllata) oil included diets, Abd Elmonem et al. (2002) when fed redtilapia the black seed (Nigella sativa) and roquette seed meals containing diets, Shalaby et al. (2003) who fed Nile tilapia licorice roots (Erksous, Glycycrhiza glabra L.) as feed aditive, Zaki and El-Ebiary (2003) when fed mono-sex Nile tilapia onion (Allium cepa) and garlic (Allium sativum) containing diets, El-Dakar (2004) by feeding hybrid tilapia on diets containing caraway seeds, El-Dakar et al. (2004a) when fed hybrid tilapia the basal leaves diets, El-Dakar et al. (2004b) who fed hybrid tilapia marjoram (Majorana hortensis) leaves included diets, El-Dakar et al. (2004c) who fed Nile tilapia the diets supplemented with fennel (Foenicum vulgare) seed meal, Bakeer and Mostafa (2006) who fed Nile tilapia chamomile flowers supplemented diets, Diab et al. (2006) when fed Nile tilapia echinacea (Echinacea purperea) and marjoram (Origanum marjorana) included diets, and Attalla (2009) by feeding Nile tilapia on ginger (Zingiber officinale) including diets. Recently, Abdelhamid and Soliman (2012a) added fenugreek seed meal (FSM) or cresson seeds meal (CSM) to Nile tilapia fry diets, they confirmed that FSM had significantly (and proportional to the increase in its addition level) improved the feed utilization in form of protein productive value and energy retention. It improved significantly also (at 2 % addition level) fish carcass protein percent. The obtained results for CSM revealed improving effect of this feed additive (particularly at 1 %) on fish growth rates (relative and specific) and feed utilization (feed protein intake, protein productive value, protein efficiency ratio, energy retention). Also, fish carcass ether extract and energy content had increased by this treatment. So, they recommended the dietary addition of 1-2 % FSM or CSM to Nile tilapia. Additionally, Abdelhamid and Soliman (2012b) fedNile tilapia fish fry dietary graded levels of guava tree leaves meal (GTLM) or camphor tree leaves meal (CTLM). The obtained results confirmed the significant gradually (with increasing the feed additive level) improvements in fish final weight, weight gain (total and daily), growth rates (relative and specific), feed utilization (feed and protein intakes, feed conversion, protein productive value, protein efficiency ratio, energy retention) by increasing GTLM or CTLM levels. These feed additives, also, significantly improved fish carcass composition (protein, ether extract, energy content). So, it could recommend the dietary inclusion of 2 % GTLM or CTLM toNile tilapia.
CONCLUSIONS
Conclusively, it could recommend the dietary addition of 1 – 2 % meals of either chmomillaflowers, majoranaleaves, ginger roots, cinnamumpark or green tealeaves, respectively, depending on their availability and prices as well as the economics of the production which need more studies.
REFERENCES
Abd El-Hakim, A. E. (2008). Effect of type of diet on productive and reproductive performance ofNiletilapia reared on commercial scale. Ph. D. Thesis, Fac. of Agric.,FayoumUniv.
Abdelhamid, A. M. (1996). Field and Laboratorial Analysis in Animal Production. Dar Annashr for Universities, Cairo, Deposit No. 11318/1996, ISBN: 977- 5526-04-1, 680 p.
Abdelhamid, A.M. (2009). Fundamentals of Fish Production and Culture. New Universal Office, Alexandria, I.S.B.N. 977–438–052–5, 393 p.
Abdelhamid, A. M. and A. A. A. Soliman (2012a). Possibility of using fenugreek seeds or cresson seeds in tilapia diets. Journal of the Arabian Aquaculture Society, 7 (1): 75 – 90.
Abdelhamid, A. M. and A. A. A. Soliman (2012b). Possibility of using dried leaves of guava and camphor trees in tilapia diets. Journal of the Arabian Aquaculture Society, 7 (1): 91 – 108.
Abd Elmonem, A. I.; Shalaby, S. M. M., and El-Dakar,A.Y. (2002). Response of red tilapia to different levels of some medical plants by-products: black seed and roquette seed meals. Proc. 1st Sci. Conf. Aqua., El-Arish, 13-15 dec., pp: 247-260.
Alsaghir, A. (2010). Cinnamum100 Foods Can Save Your Life. Shoa'a for Publication and Sciences,Halab,Syria.
Aly, S.M., Abdel Atti, N.M.and Mohamed, M.F. (2008). Effect of garlic on the survival, growth, resistance and quality of Oreochromis niloticus. Proceeding of the InternationalSymposium on Tilapia in Aquaculture. Pp: 277 – 296.
Anon. (2010). The Tea Guardian. Green Teas: A very brief history. http://www.almania.info/9H, Anon. (2013). Owered by vBulletin® Version Copyright ©2000 - 2013, vBulletin Solutions, Inc
A.O.A.C. Association of Official Agricultural Chemists (1990). Official methods of analysis. 15th Ed. Published by the A.O.A.C., Benjamin Francklin Station, Washington. D.C., USA.
Attalla, R. F. (2009). Growth response and physiological activities of the Niletilapia (Oreochromis niloticus) fed basal diets supplemented with ginger (Zingiber officinale) as natural growth promoters.Egypt. J. Aqut. & Fish., 13 (4): 85-107.
Bakeer , M.N. and M. A. A. Mostafa (2006). The importance of betafin or chamomile flower as natural feed additives forNiletilapia fry cultured under cold season conditions. J. Agric. Sci. Mansoura Univ., 31: 4145-4153.
Diab, A.S., Abdel-Hadi, Y.M., Ahmad, M.H., Sakr, S.F. and Abo el-Atta, M.E. (2006). Outdoor study on the use of echinacea (Echinacea purperea), marjoram (Origanum majorana) and yeast (Saccharomyces cerevisiae) as feed additives for Oreochromis niloticus. Egypt. J. Agric. Res., 84 (1 B): 537 – 551.
Duncan, D.B. (1955). Multiple ranges and multiple F-tests. Biometrics, 11: 1-42.
El-Dakar, A. Y. (2004). Growth response of hybrid tilapia, Oreochromis niloticus x Oreochromis aureus, fingerlings to diets supplemented with different levels of caraway seeds. J. Agric. Sci. Mansoura Univ., 29 (11): 6083-6094.
El-Dakar, A. Y.; Hassanien, G. D., Gad, S. S. and Sakr, S. E. (2004a). Medicinal and aromatic plants as feeding attractants for fish. II. Effect of dried basil leaves on performance of hybrid tilapia, Oreochromis niloticus x Oreochromis auraus, fingerlings. 3rd Con. on Anim. Poul. & Fish Prod. & Health in Semi-Arid Areas, El-Arish, 7-9 Sept., pp: 265-277.
El-Dakar, A. Y.; Hassanien, G. D., Gad, S. S. and Sakr, S. E. (2004b). Medicinal and aromatic plants as feeding attractants for fish. I. Effect of dried marjoram leaves on performance of hybrid tilapia, Oreochromis niloticus x Oreochromis auraus, fingerlings. J. Egypt. Acad. Soc. Environ. Develop., (B-Aquaculture), 5 (1): 67-83.
El-Dakar, A. Y.; Shalaby, S. M. M., Abd Elmonem, A. I. and Wahbi, O. M. (2004c). Enhancement of growth performance of Oreochromis niloticus using fennel seed meal as feed additive. J. Egypt. Acad. Soc. Environ. Develop., (B-Aquaculture), 5 (2): 43-67.
El-Saidy, D.M.S.D. and Gaber, M.M.A. (1997). Effect of different levels of dry garlic meal supplemented to the diets on growth performances, survival and body composition of nile tilapia (Oreochromis niloticus) fingerlings. Annals of agric. Sc., moshtohor, 35 (3): 1197-1209.
Gaber, M. M. (2000). Growth response of Nile tilapia fingerlings (Oreochromisniloticus) fed diets containing different levels of clove oil. Egypt. J. Aquat. Biol. & Fish., 4 (1): 1-18.
Ibrahim, M. (2013). Marjoram.NationalResearchCenter,Cairo.
Macdonald, P., Edwards, R.A. and Greenhalgh, J.F.D. (1973). Animal Nutrition, 2nd Ed., Longman,London.
Pantha, B. (1982). The use of soybean in practical feeds for Tilapia niloticus. M. Sc. Thesis. Univ. of Stirling.
SAS (1996). SAS/STAT Guide for personal computer. SAS Inst. Cary, N. C.
Shalaby, S.M.M., Abd Elmonem, A.I., El-Dakar, A.Y. and Wahbi, O.W. (2003). Improvement of growth and feed utilization by using licorice roots (Erksous) as a feed additive in diets of Nile tilapia, Oreochromis niloticus fingerlings. J. Egypt. Acad. Soc. Environ. Develop., (B- Aquaculture), 4 (2): 119 – 142.
Tawfik, S.A, Ahmed, M.E., Saeta, E.I. and El-Gazzar, O.B. (2005). Effect of adding chamomile flowers to aflatoxins containing diet on growth performance, rumen parameters and some blood components of growing lambs. 4th Inter. Sci. Conf. Mansoura.
USDA (2008). Nutrient database and Zingiber officinale information from NPGS/GRIN. www.ars-grin.gov.
Zaki, M. A. and El-Ebiary, E. H. (2003). Effect of incorporation of onion and garlic into diets on growth performance and body composition of mono-sex Niletilapia (Oreochromis niloticus).Egypt. J. Aquat. Biol. & Fish., 7 (1): 113-126.






.jpg&w=3840&q=75)