Studies point to plasma as safe feed ingredient
Published: August 26, 2014
Source : North American Spray Dried Blood Products Producers (NASDBPP) association
PORCINE epidemic diarrhea virus (PEDV) is difficult to control and causes high death loss in suckling pigs younger than two weeks of age, resulting in signifi cant financial loss to all sectors of the swine industry. PEDV spreads quickly and easily.
The primary route of infection is through direct contact with infected pigs or from the manure of infected pigs. Other routes of infection responsible for spreading the virus may be contaminated transport vehicles, farm equipment and farm workers and visitors.
Industry leaders are actively discussing and reviewing data concerning the role feed and feed ingredients play in the spread of PEDV. Speculation that PEDV is spread by feed has led to implementation of costly biosecurity programs, often with little controlled research or data supporting the necessity or effectiveness of such a program.
The first report of PEDV in Ontario and subsequent investigation resulted in the belief that nursery feed containing porcine plasma may have been the source of the PEDV infections.
The Canadian Food Inspection Agency (CFIA) reported that infective virus was detected in samples of porcine plasma, but infective virus could not be detected in the feed containing the porcine plasma. Even with this conflicting data, many industry professionals concluded that spray-dried porcine plasma spreads the disease.
In addition, despite the long history of indisputable performance benefits, some industry professionals have recommended removing spray-dried porcine plasma and, in some cases, all porcine-based ingredients from feed for swine.
Epidemiology is a powerful scientific tool that can be used to identify associations of exposure to health outcomes. Epidemiologic observation allows scientists to form a hypothesis and then test the hypothesis in controlled experiments.
Experiments
Experiment 1. The effect of spray drying conditions on the survival of PEDV.
Hypothesis: If spray-dried porcine plasma contains infective virus and is a vector for spreading PEDV, then the virus will survive the spray-drying process.
Liquid bovine plasma was inoculated with PEDV (Strain CV777) to a final TCID50 (median tissue culture infective dose) concentration of 104.2 per milliliter of liquid plasma. Three aliquots of the PEDV-inoculated plasma were spray dried using a laboratory scale Buchi 190 Mini Spray Dryer at an inlet temperature of 200°C.
In order to simulate manufacturing conditions for commercial spray-dried plasma, the product was processed to achieve an inner temperature of 70°C or 80°C throughout its substance, respectively. The normal outlet temperature of product at commercial manufacturing plants is 80°C throughout its substance. The test also included samples processed with a lower outlet temperature to determine if PEDV could remain active at 70°C. Both liquid and spray-dried samples were analyzed for PEDV infectivity in VERO cell monolayers using a micro-titer assay procedure and were subjected to three consecutive serial passages to new VERO cell culture.
PEDV strain CV777 was used in this study because it is known to multiply on VERO cells to a high enough concentration to determine inactivation by spray drying.
The results (Table 1) show that PEDV is inactivated by spray drying at temperatures even lower than those used for commercial production of spray-dried plasma. These results do not support the conclusion that spray-dried porcine plasma contains infective PEDV and is a vector in spreading PEDV.
Experiment 2a. Bioassays and feeding trials designed to determine if spraydried porcine plasma that tests positive for PEDV contains an infective virus.
Hypothesis: If spray-dried porcine plasma contains infective virus and is a vector for spreading PEDV, then the retained samples of spray-dried plasma samples being investigated by CFIA should also contain infective PEDV.
Because the spray-dried plasma product investigated by CFIA was produced in the U.S., CFIA requested that the Food & Drug Administration conduct an investigation. FDA inspected the U.S. spray-dried plasma manufacturing plant, reviewed production records (including the production records of the spray-dried plasma CFIA investigated) and collected retained samples of the specific lot of spray-dried plasma in question.
The production records confirmed that the specific lot of spray-dried plasma was produced within normal operating standards.
Independently, both FDA and NASDBPP submitted the retained spray-dried plasma samples for further analysis, including pig bioassays. FDA recently released the results of its pig bioassays (Table 2) in which three retained samples from the lot of plasma being investigated by CFIA were tested for the presence of infective PEDV. All three spray-dried plasma samples were negative for PEDV infectivity by bioassay.
In addition, NASDBPP conducted additional pig bioassays on spray-dried porcine plasma. Two samples from the same lot investigated by CFIA and two samples from randomly selected production lots were tested. All four samples of spray-dried plasma were negative for PEDV infectivity by bioassay (Table 3).
In contrast to the results reported by CFIA, FDA and NASDBPP were unable to detect infective PEDV in retained samples of porcine plasma. It is important to note that the retained samples were stored under the same conditions as the commercial product. However, CFIA collected the plasma samples from the fi eld after the plasma had been picked up by the customer and delivered to the feed mill, while the retained samples were maintained in the spray-drying plant warehouse.
These results do not support the conclusion that spray-dried porcine plasma contains infective PEDV and is a vector for spreading PEDV.
Some in the industry have questioned the sensitivity of pig bioassays because they involve a small number of animals fed a limited amount of test ingredient. Therefore, it is diffi cult to detect a low occurrence event, and a negative does not necessarily mean negative.
In an attempt to address this concern, two additional trials were designed with feeding protocols refl ecting industry practices.
Experiment 2b. Hypothesis: If spraydried porcine plasma contains infective virus and is a vector for spreading PEDV, then feeding plasma-containing diets for longer periods of time, similar to commercial practice, will increase the chance of detecting the presence of infective virus.
Feeding trial 1: At a contract research facility that had previously housed nursery pigs tested and found negative for PEDV, 48 pigs weaned at an average of 21 days old were randomly distributed into two different treatment groups, with six pens per dietary treatment and four pigs per pen.
Pigs in the treatment 1 group received a control diet without spray-dried porcine plasma. Pigs in the treatment 2 group received a meal diet containing 5% commercial spray-dried porcine plasma (with a cycles to threshold [Ct] of 26.2) that was polymerase chain reaction (PCR) positive for PEDV (Ct of complete feed was 30.1).
Basal diets were similar to standard commercial diets for weaned pigs, and the 5% spray-dried porcine plasma included in treatment 2 replaced soy protein concentrate on an equal lysine basis. The spray-dried porcine plasma used in the test diet had the lowest Ct result of commercial product produced during 2014. Experimental diets were fed in a meal form (eliminating the confounding heat treatment associated with pelleting) for 14 days after weaning, and then all pigs were fed the control diet for an additional seven days.
Pigs were evaluated daily for clinical symptoms of both enteric and respiratory diseases. Pens and pigs were evaluated daily and assigned a score for diarrhea. Rectal fecal swabs were collected from each pig at days 0, 3, 7, 14 and 21 and were immediately submitted for PEDV PCR analysis.
Pigs were necropsied on day 21. Gross evaluation of tissues was conducted to determine if any abnormalities were noted. Intestinal contents were collected at termination and submitted for PEDV PCR, and tissue samples were subjected to immunohistochemistry. Terminal blood samples were collected and submitted for PEDV antibody analysis.
In this experiment, feeding pigs a diet containing 5% commercial spray-dried porcine plasma that was PCR positive for PEDV did not demonstrate any evidence of PEDV infectivity in these pigs through 21 days postweaning (Table 4).
Feeding trial 2: At Iowa State University, 21-day-old weaned pigs confi rmed negative for PEDV were randomly allotted to four treatment groups as follows:
- Treatment 1 pigs were not inoculated with PEDV and were fed a control mealform diet without spray-dried porcine plasma.
- Treatment 2 pigs were not inoculated with PEDV and were fed a meal-form diet with 5% spray-dried porcine plasma that was PCR positive for PEDV (Ct = 30.0).
- Treatment 3 pigs were inoculated with PEDV and fed the control diet.
- Treatment 4 pigs were inoculated with PEDV and fed the diet containing 5% spray-dried porcine plasma that was PCR positive for PEDV (Ct = 30.0).
Diets for treatments 1 and 2 were fed for 28 days postweaning, while diets for treatments 3 and 4 were provided to pigs four days before PEDV (isolate 13-19338E) inoculation and then were fed for an additional 28 days. Six pigs were allotted per treatment. Three pigs per group were necropsied on days 7 and 28 of the study.
Regardless of the diet fed, pigs in treatments 1 and 2 not inoculated with PEDV did not develop PEDV, as determined by PCR of fecal swab samples or by the development of serum antibodies against PEDV (Table 5). Feeding non-inoculated pigs a diet with PEDV PCR-positive spray-dried porcine plasma did not result in PEDV infectivity over the 28-day study. All pigs in treatments 3 and 4 inoculated with PEDV, regardless of diet fed, had PCR positive fecal samples starting at day 3 post-inoculation through day 28 and had developed serum antibodies against PEDV.
The data from these two studies demonstrate that pigs fed a diet containing spray-dried porcine plasma that’s PCR positive for PEDV, simulating commercial feeding programs, did not become infected with PEDV. These results do not support the conclusion that spraydried porcine plasma contains infective PEDV and is a vector for spreading PEDV.
Experiment 3. Stability trials designed to determine the survival of PEDV on dry plasma stored at different temperatures. Hypothesis: If spray-dried porcine plasma contains infective virus and is a vector for spreading PEDV, then PEDV should survive on spray-dried plasma long enough to move through the commercial feed channel.
Spray-dried bovine plasma was inoculated with PEDV strain CV777 to a final concentration of 103 TCID50 per gram and maintained at room temperature (20-22°C) for one week. Initial and fi nal samples were assayed in VERO cell culture as previously described.
At seven days, PEDV infectivity was not detected in the inoculated spray-dried bovine plasma (Table 6). In summary, PEDV did not survive on spray-dried plasma by seven days when stored at room temperature.
Based on the results from the initial experiment, a follow-up experiment was designed to understand the effects of storage time at various temperatures on PEDV survival on spray-dried plasma. In this experiment, spray-dried bovine plasma was externally contaminated with PEDV and stored at 4°C, 12°C and 22°C. PEDV isolation, as described previously, was determined at days 0, 7, 14 and 21 post-inoculation, representing five samples per time by temperature storage condition.
Summary. At a 4°C storage temperature, PEDV survived on spraydried plasma for 14 days but did not survive when stored for 21 days (Table 7); at a 12°C storage temperature, PEDV survived on spray-dried plasma for seven days but did not survive when stored for 14 or 21 days, and at a 22°C room temperature storage, PEDV did not survive on spray-dried plasma for 7, 14 or 21 days.
PEDV does not survive for extended periods of time on spray-dried plasma. In two experiments, PEDV survived fewer than seven days when held at room temperature (71°F). Even at refrigerated temperatures, PEDV survived fewer than 21 days. Typically, spray-dried plasma will be held for a minimum of one week before testing is completed and the product is released for sale. Depending on inventory, another two weeks will be required for product to be sold, picked up, delivered and mixed into complete feed.
In addition, these data support the redundant safety step introduced by NASDBPP of holding spray-dried plasma for 14 days at 71°F before it is released for sale.
These results do not support the conclusion that spray-dried porcine plasma contains infective PEDV and is a vector for spreading PEDV.
Typically, spray-dried plasma is stored in an unheated warehouse before shipment to the feed manufacturer. Because of the exceptionally cold winter of 2013-14, it was speculated that spraydried plasma was essentially held in a “deep freeze,” effectively extending the survival of PEDV and, therefore, allowing spray-dried plasma to spread PEDV.
NASDBPP conducted the following study to understand actual storage conditions in warehouse systems. However, the data from the study indicated that this speculation is incorrect.
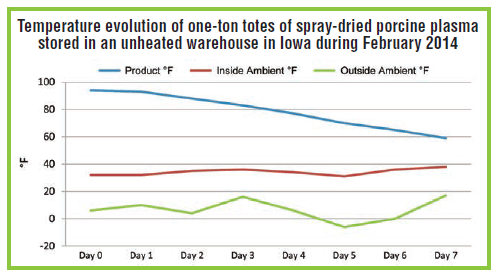
During late February 2014, the temperature of spray-dried porcine plasma was measured during packaging and daily while the product was held for quality assurance analysis (Figure). The warehouse, located in central Iowa, was not insulated or heated. The outside temperatures ranged between -6°F and 17°F, while the warehouse temperature fl uctuated between 27°F and 37°F.
During packaging, the spray-dried plasma was approximately 95°F and slowly cooled to 59°F within seven days. The product would not be expected to cool below the warehouse temperature. Therefore, even in an uninsulated warehouse during the exceptionally cold 2013-14 winter, spray-dried plasma did not experience a deep freeze; actually, it maintained temperatures shown to inactivate PEDV.
Experiment 4. Incidence of PEDV in distinct regional swine populations that consumed significant quantities of spraydried porcine plasma that was positive for PEDV. Hypothesis: If spray-dried porcine plasma contains infective virus and is a vector for spreading PEDV, then feeding spray-dried porcine plasma PCR positive for PEDV should spread the virus to PEDV-free regions.
A spray-drying facility located in the southern region of Brazil is dedicated to porcine blood processing only. Samples of spray-dried porcine blood were collected from production lots dried in late December 2013 and early January 2014. The samples were submitted for PCR analysis for the presence of the PEDV genome.
The samples were negative (Table 8), confi rming the observations of veterinarians in Brazil that PEDV has not entered Brazil. During 2013, enough U.S.- produced spray-dried porcine plasma (positive for PEDV) was exported to Brazil to feed 2.5-3.5 million pigs. Despite this large quantity of U.S. PEDV PCRpositive spray-dried porcine plasma being fed in Brazil, PEDV has not been diagnosed or reported in this swine population.
Additionally, there’s a spray-drying facility in western Canada dedicated to porcine blood processing only. Samples of spray-dried porcine plasma were submitted for PCR analysis for the presence of the PEDV genome, and all samples analyzed over the last several months were negative (Table 9).
Between April and December 2013, enough PEDV PCR-positive spray-dried porcine plasma was exported from U.S. spray-dried plasma manufacturing plants into western Canada to feed 3.5-4.0 million pigs, yet no PEDV cases were detected in western Canada during that time period. The pig population in western Canada remained negative for PEDV through March 2014, with the exception of one case reported in February that was determined to be unrelated to feed.
Data from controlled experiments and regional observations from feeding U.S.- sourced PCR-positive spray-dried porcine plasma to 2.5-3.5 million pigs in Brazil and 3.5-4.0 million pigs in western Canada do not support the conclusion that spraydried porcine plasma contains infective PEDV and is spreading the disease.
Conclusion
Spray-dried plasma is a safe, effective feed ingredient. It contains a diverse mixture of proteins that provide nutrition to support benefi cial and functional biological effects on pigs’ ability to thrive and cope with postweaning stress.
The nutrition provided by including spray-dried plasma as a key ingredient in nursery diets has been recognized by the swine industry worldwide because of its well-documented benefi cial effects on postweaning growth, feed intake, morbidity indices and survival.
In fact, results of many studies conclude that supplementing diets with spray-dried plasma supports and maintains swine under challenge with porcine circovirus-2 associated diseases, porcine reproductive and respiratory syndrome and other respiratory and gastrointestinal diseases, including transmissible gastroenteritis virus. Therefore, it can be expected that feed supplemented with spray-dried plasma has the potential to support pigs’ ability to cope with PEDV.
The recent research and study results outlined herein demonstrate that PEDV is inactivated during commercial production of spray-dried plasma and that PEDV PCR-positive spray-dried porcine plasma is not a source of PEDV infectivity. These results confi rm evidence from past research that spraydried porcine plasma is a safe and vital feed ingredient for the global swine industry.
Source
North American Spray Dried Blood Products Producers (NASDBPP) associationRelated topics:
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.





