Therapeutic Supplementation of Caprylic Acid in Feed Reduces Campylobacter jejuni Colonization in Broiler Chicks
Published: October 17, 2013
By: F. Solis de los Santos,1 A. M. Donoghue,2 K. Venkitanarayanan,3 I. Reyes-Herrera,1 J. H. Metcalf,1 M. L. Dirain,1 V. F. Aguiar,1 P. J. Blore,1 and D. J. Donoghue1*
1Poultry Science Department, University of Arkansas, Fayetteville, Arkansas 72701
2 Poultry Production and Product Safety Research Unit, Agricultural Research Service, USDA, Fayetteville, Arkansas 72701
3 Department of Animal Science, University of Connecticut, Storrs, Connecticut 06269
2 Poultry Production and Product Safety Research Unit, Agricultural Research Service, USDA, Fayetteville, Arkansas 72701
3 Department of Animal Science, University of Connecticut, Storrs, Connecticut 06269
Appl. Environ. Microbiol. 2008, 74(14):4564. DOI:10.1128/AEM.02528-07.
Publication date: Received 8 November 2007/Accepted 12 May 2008
Campylobacter species are some of the most commonly reported bacterial causes of human food-borne illnesses in the United States, and epidemiological evidence indicates that poultry and poultry products are significant sources of human infection (6, 10, 18). Contamination with Campylobacter originates from environmental sources, including flies, rodents, and wild birds (11, 17), and spreads rapidly through the flock (14). Even with biosecurity measures, Campylobacter colonization is widespread in most poultry flocks (4, 15, 29).
Cecal carriage of C. jejuniresults in horizontal transmission of the pathogen and in carcass contamination during slaughter. Therefore, interventional strategies implemented at the farms for reducing C. jejuni counts in the chicken intestinal tract are critical for delivering a microbiologically safer product. Fatty acids, especially medium chain fatty acids, were found to have antimicrobial properties for a wide range of microorganisms (2, 3, 16, 19, 24, 27). Recently, Thormar and coworkers (26) reported that monocaprin, the monoglyceride of capric acid, was effective in killing significant populations of C. jejuni in chicken feed. Caprylic acid is a medium chain fatty acid with eight carbons naturally found in breast milk, bovine milk, and coconut oil (12, 13, 16). It is a food-grade compound classified as generally recognized as safe (GRAS) by the U.S. Food and Drug Administration. We recently demonstrated the prophylactic efficacy of feed supplemented with caprylic acid against Campylobacter colonization in 10-day-old broiler chicks (23). This strategy provides a potentially important method for limiting Campylobacter colonization; however, strategies are needed to reduce Campylobacter populations after colonization in poultry has already occurred. Therefore, the objective of the present study was to determine the therapeutic effect of feed supplemented with caprylic acid on C. jejuni colonization in broiler chicks.
Experimental design. In three separate trials, day-of-hatch commercial broiler chicks (n = 60 per trial; mixed sex) were assigned to six treatment groups (n = 10 per group): negative controls (no Campylobacter; no caprylic acid); positive controls (Campylobacter; no caprylic acid); and four caprylic acid treatment groups (based on caprylic acid doses of 0.35%, 0.7%, 1.4%, and 2.8%). These doses were selected since all doses were equally effective at killing Campylobacter within 1 h in vitro (data not shown). Caprylic acid (Sigma-Aldrich, St. Louis, MO) was supplemented in starter feed for the last 72 h of each 15-day trial. At 3 days of age, chicks were inoculated with five wild-type strains of C. jejuni (8), and at 15 days of age, chicks were euthanized, and cecal contents were collected for Campylobacter enumeration (7). Birds were individually weighed on days 12 and 15 to determine body weight differences, feed consumption, and feed conversion during the treatment period. There was no morbidity or mortality during the treatment period.
Based on the results from trials 1 and 2, intestinal samples from chicks fed with 0.7% and 1.4% caprylic acid and the positive control (0% caprylic acid) were collected for enteric morphometric analysis in trial 3 on day 15 of the experiment. Segments (2 cm) of the midpoint of the duodenum, jejunum, ileum, and cecum were prepared and evaluated (22). Twenty replicates for each variable studied (villus height, base, surface area, crypt depth, lamina propria thickness, and neutral goblet cell density) were measured for each sample. Data were analyzed by analysis of variance, using the GLM procedure of SAS, with means partitioned by LSMEANS analysis (STAT user's guide, release version 9.03; SAS Institute Inc., Cary, NC).
The results of this study demonstrate that select doses of caprylic acid, when fed for only 3 days, can consistently reduce enteric Campylobacter populations in young chickens already colonized with the bacterium (Fig. 1). In three separate trials, supplementation of a 0.7% and a 1.4% dose of caprylic acid reduced cecal Campylobacter counts substantially compared with those in the positive controls. Supplementation of caprylic acid at 0.35% and 2.8% levels had an inconsistent effect on the reduction of C. jejuni populations in the cecal content.
FIG. 1. Effect of caprylic acid on cecal C. jejuni counts in 15-day-old chickens. Values (means ± standard errors of the means) per treatment (n = 10 birds/treatment per trial) represent the population of C. jejuni in the cecal content of 15-day-old broiler chickens. In each trial, chicks (except for negative controls) were orally challenged 3 days posthatch with a mixture of five C. jejuni isolates (n = 10 chicks/treatment per trial; average dose of 2 x 106 CFU/ml). Caprylic acid was added to the feed at 72 h prior to necropsy. Columns within the same trial (1 to 3) with different letters (a, b, c, and d) denote significant differences between doses (P < 0.05). All Campylobacter data were log10 transformed for statistical analysis (5).
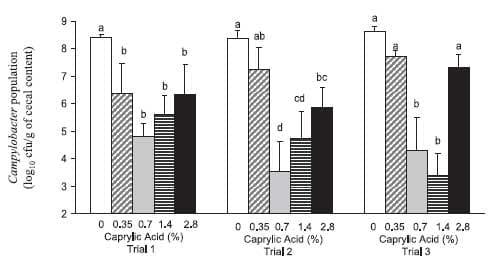
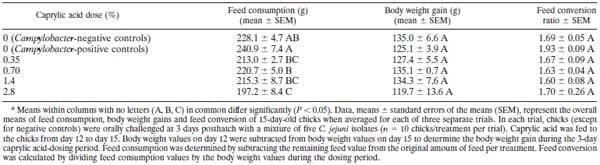
Since caprylic acid is a natural and GRAS-designated feed additive, it could be used immediately by poultry farmers to reduce C. jejuni carriage in chickens. Using the 0.7 and 1.4% doses, we observed a consistent 3- to 4-log reduction in cecal Campylobacter populations in chicks. This could have significant beneficial implications for food safety, since, during processing, enteric contents can contaminate the carcass, thereby resulting in food-borne transmission of C. jejuni . (1, 21). It has been estimated that a 2-log reduction in C. jejuni populations on poultry carcass contaminations could bring about a 30-fold reduction in human Campylobacteriosis cases (20). The use of caprylic acid should be accepted by poultry producers, since it does not have any adverse effect on parameters important for production (profit) or health such as morbidity, mortality, body weight gain, or feed conversion (Table 1). The cost of treatment would be limited ($2 to 3/kg; KIC Chemicals Inc., New Paltz, NY) because it has therapeutic efficacy and would be used only for the last 3 days prior to slaughter. This dosing strategy would have the added benefit of preventing the reestablishment of higher C. jejuni populations, since there would be no withdrawal period prior to slaughter.
TABLE 1. Effect of caprylic acid on feed consumption, body weight gain, and feed conversion in chicksa
The mechanism of caprylic acid-mediated Campylobacter reduction in chicks is not clear but may be due to the diffusion of caprylic acid into bacterial cells in the undissociated form and dissociation within the protoplasm, thereby leading to intracellular acidification (25). A lower intracellular pH can lead to inactivation of intracellular enzymes (28) and inhibition of amino acid transport (9). Based on previous morphometric analysis, we postulated that another possible mechanism of action of caprylic acid may be a physical or functional alteration of the gastrointestinal colonization site of C. jejuni in chicks. Our previous research with poults fed bacteriocins found that the reduction in Campylobacter counts was associated with an alteration in the preferential gastrointestinal colonization sites for this organism (7). In the present study, caprylic acid supplementation showed either no effects or inconsistent effects on gastrointestinal morphology (data not shown), suggesting that other mechanisms are responsible for the reduction observed for cecal C. jejuni counts. Caprylic acid may compromise the outer membrane determinants in Campylobacter bacteria that are needed for bacterial adaptation to the host environment and colonization, or it may have a direct inhibitory effect on the expression of virulence factors necessary for C. jejuni colonization. However, additional research is necessary to confirm these hypotheses and to determine why the highest dose of caprylic acid (2.8%) was not consistently effective. It is possible that higher doses of caprylic acid alter competing intestinal microflora, allowing higher Campylobacter carriage.
In conclusion, the results of the present study suggest that therapeutic supplementation of caprylic acid for 3 days reduced Campylobacter populations in the cecal content of chicks by 3 to 4 logs. Follow-up studies are currently under way to elucidate the mechanism(s) by which caprylic acid reduces enteric Campylobacter carriage in poultry.
This work was supported in part by USDA, CSREES National Integrated Food Safety Program grant no. 2006-02429 to K. Venkitanarayanan and D. J. Donoghue.
Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the USDA and does not imply its approval to the exclusion of other products that are suitable.
REFERENCES
Allen, V. M., S. A. Bull., J. E. Corry, G. Domingue, F. Jørgensen, J. A. Frost, R. Whyte, A. Gonzalez, N. Elviss, and T. J. Humphrey. 2007. Campylobacter spp. contamination of chicken carcasses during processing in relation to flock colonization. Int. J. Food Microbiol. 113:54–61.
Annamalai, T., M. K. Mohan Nair, P. Marek, P. Vasudevan, D. Schreiber, R. Knight, T. Hoagland, and K. Venkitanarayanan. 2004. In vitro inactivation of Escherichia coli O157:H7 in bovine rumen fluid by caprylic acid. J. Food Prot. 67:884–888.
Bergsson, G., J. Arnfinnsson, S. M. Karlsson, O. Steingrimsson, and H. Thormar. 1998. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob. Agents Chemother. 42:2290–2294.
Berrang, M. E., R. J. Buhr, and J. A. Cason. 2000. Campylobacter recovery from external and internal organs of commercial broiler carcasses prior to scalding. Poult. Sci. 79:286–290.
Byrd, J. A., R. C. Anderson, T. R. Callaway, R. W. Moore, K. D. Knape, L. F. Kubena, R. L. Ziprin, and D. J. Nisbet. 2003. Effect of experimental chlorate product administration in the drinking water on Salmonella typhimurium contamination of broilers. Poult. Sci. 82:1403–1406.
Centers for Disease Control and Prevention. 2007. Preliminary FoodNet data on the incidence of infection with pathogens transmitted commonly through food: 10 states, United States, 2006. MMWR Morb. Mortal. Wkly. Rep. 56:336–339.
Cole, K., M. B. Farnell, A. M. Donoghue, N. J. Stern, E. A. Svetoch, B. N. Eruslanov, L. I. Volodina, Y. N. Kovalev, V. V. Perelygin, E. V. Mitsevich, I. P. Mitsevich, V. P. Levchuk, V. D. Pokhilenko, V. N. Borzenkov, O. E. Svetoch, T. Y. Kudryavtseva, I. Reyes-Herrera, P. J. Blore, F. Solis de los Santos, and D. J. Donoghue. 2006. Bacteriocins reduce Campylobacter colonization and alter gut morphology in turkey poults. Poult. Sci. 85:1570– 1575.
Farnell, M. B., A. M. Donoghue, K. Cole, I. Reyes-Herrera, P. J. Blore, and D. J. Donoghue. 2005. Campylobacter susceptibility to ciprofloxacin and corresponding fluoroquinolone concentrations within the gastrointestinal tracts of chickens. J. Appl. Microbiol. 99:1043–1050.
Freese, E., C. W. Sheu, and E. Galliers. 1973. Function of lipophilic acids as antimicrobial food additives. Nature 241:321–325.
Friedman, C. R., R. M. Hoekstra, M. Samuel, R. Marcus, J. Bender, B. Shiferaw, S. Reddy, S. D. Ahuja, D. L. Helfrick, F. Hardnett, M. Carter, B. Anderson, and R. V. Tauxe. 2004. Risk factors for sporadic Campylobacter infection in the United States: a case-control study in FoodNet sites. Clin. Infect. Dis. 38:S285–296.
Gregory, E., H. Barnhart, D. W. Dreesen, N. Stern, and J. Corn. 1997. Epidemiological study of Campylobacter spp. in broilers: source, time of colonization, and prevalence. Avian Dis. 41:890–898.
Jensen, R. G., A. M. Ferris, C. J. Lammi-Keefe, and R. A. Henderson. 1990. Lipids of bovine and human milks: a comparison. J. Dairy Sci. 73:223–240.
Jensen, R. G. 2002. The composition of bovine milk lipids: January 1995 to December 2000. J. Dairy Sci. 85:295–350.
Lee, M. D., and D. G. Newell. 2006. Campylobacter in poultry: filling an ecological niche. Avian Dis. 50:1–9.
Loc Carrillo, C., R. J. Atterbury, A. El-Shibiny, P. L. Connerton, E. Dillon, A. Scott, and I. F. Connerton. 2005. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 71:6554–6563.
Nair, M. K., J. Joy, P. Vasudevan, L. Hinckley, T. A. Hoagland, and K. S. Venkitanarayanan. 2005. Antibacterial effect of caprylic acid and monocaprylin on major bacterial mastitis pathogens. J. Dairy Sci. 88:3488–3495.
Newell, D. G., and C. Fearnley. 2003. Sources of Campylobacter colonization in broiler chickens. Appl. Environ. Microbiol. 69:4343–4351.
Oosterom, J., C. H. den Uyl, J. R. J. Banffer, and J. Huisman. 1984. Epidemiological investigations on Campylobacter jejuni in households with primary infection. J. Hyg. (London) 92:325–332.
Petschow, B. W., R. P. Batema, and L. L. Ford. 1996. Susceptibility of Helicobacter pylori to bactericidal properties of medium-chain monoglycerides and free fatty acids. Antimicrob. Agents Chemother. 40:302–306.
Rosenquist, H., N. L. Nielsen, H. M. Sommer, B. Norrung, and B. B. Christensen. 2003. Quantitative risk assessment of human Campylobacteriosis associated with thermophilic Campylobacter species in chickens. Int. J. Food Microbiol. 83:87–103.
Rosenquist, H., H. M. Sommer, N. L. Nielsen, and B. B. Christensen. 2006. The effect of slaughter operations on the contamination of chicken carcasses with thermotolerant Campylobacter. Int. J. Food Microbiol. 108:226–232.
Solis de los Santos, F., A. M. Donoghue, M. B. Farnell, G. R. Huff, W. E. Huff, and D. J. Donoghue. 2007. Gastrointestinal maturation is accelerated in turkey poults supplemented with a mannan-oligosaccharide yeast extract (Alphamune). Poult. Sci. 86:921–930.
Solis de los Santos, F., A. M. Donoghue, K. Venkitanarayanan, M. L. Dirain, I. Reyes-Herrera, P. J. Blore, and D. J. Donoghue. 2008. Caprylic acid supplemented in feed reduces Campylobacter jejuni colonization in ten-dayold broiler chickens. Poult. Sci. 87:800–804.
Sprong, R. C., M. F. Hulstein, and R. van Der Meer. 2001. Bactericidal activities of milk lipids. Antimicrob. Agents Chemother. 45:1298–1301.
Sun, C. Q., C. J. O'Connor, S. J. Turner, G. D. Lewis, R. A. Stanley, and A. M. Roberton. 1998. The effect of pH on the inhibition of bacterial growth by physiological concentrations of butyric acid: implications for neonates fed on suckled milk. Chem. Biol. Interact. 113:117–131.
Thormar, H., H. Hilmarsson, and G. Bergsson. 2006. Stable concentrated emulsions of the 1-monoglyceride of capric acid (monocaprin) with microbicidal activities against the food-borne bacteria Campylobacter jejuni, Salmonella spp., and Escherichia coli. Appl. Environ. Microbiol. 72:522–526.
Vasudevan, P., M. Patrick, M. Nair, T. Annamalai, M. Darre, M. Khan, and K. Venkitanarayanan. 2005. In vitro inactivation of Salmonella enteritidis in autoclaved chicken cecal contents by caprylic acid. J. Appl. Poult. Res. 14:122–125.
Viegas, C. A., and I. Sa-Correia. 1991. Activation of plasma membrane ATPase of Saccharomyces cerevisiae by octanoic acid. J. Gen. Microbiol. 137:645–651.
Wallace, J. S., K. N. Stanley, and K. Jones. 1998. The colonization of turkeys by thermophilic Campylobacters. J. Appl. Microbiol. 85:224–230.
Ver email adjunto. El archivo es Therapeutic...
Related topics
Authors:
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post







.jpg&w=3840&q=75)