Safety and efficacy of a vector Marek’s/Newcastle vaccine (rHVT-F) applied to broilers reared in non-vaccinating and velogenic Newcastle Disease virus (NDV)-free poultry production systems
Published: September 2, 2016
By: Luiz Sesti 1, Carlos Kneipp 2, Leandro Bianchet 2.
/ Ceva Animal Health - 1 Latin America, 2 Brazil.
Introduction
Velogenic Newcastle Disease (ND), a dreadful poultry disease caused by a paramyxovirus type-I (Newcastle Disease Virus - NDV), continues to be an important threat to the modern poultry industry all over the world (4). An effective control of ND consists primarily of good biosecurity practices, preventive vaccination of flocks and the culling of infected birds and birds at risk of being infected. Vaccination programs are designed to suit the prevailing ND epidemiological situation and take into account other factors such as maternal immunity, additional vaccination programs, other pathogen circulation, types of flock, available labor, climatic conditions, inferred cost and type of vaccine used (1,4,8).
The main goal of this work was to investigate the field safety and efficacy of vaccinating against ND broilers reared in non-vaccinating and velogenic ND-free production systems. Companies involved in the field trial are located in the southeastern and southern region of Brazil and have not vaccinated their commercial broilers against ND for more than 20 years.
Materials and methods
Testing vaccine
Commercial broilers were vaccinated with a recombinant turkey herpesvirus-based vector vaccine (rHVT) expressing a key protective antigen (F glycoprotein) of the NDV (Vectormune® ND - Ceva Animal Health - Lenexa, Kansas, USA – hereinafter referred to as rHVT-F).
Experimental groups
The data to be presented are from two large poultry companies (integrated production systems) located in the southern region of Brazil.
Company 1:
Company not vaccinating its commercial broilers against ND for more than 20 years.
A large number of broiler (943,000) were vaccinated (day one/SQ) against ND with the vector vaccine rHVT-F and their seroconversion and growth and clinical performance compared with that of contemporary broilers not vaccinated against ND (902,000).
The objective of the use of the vector Marek’s/Newcastle (rHVT-F) vaccine was to assess its field safety and induced seroconversion in a totally ND-free epidemiological situation.
Company 2:
Company not vaccinating their commercial broilers against ND for more than 20 years as well.
The growth and clinical performance and broilers (298,000) not vaccinated against ND was compared with those of contemporary broilers (285,000) vaccinated (in ovo) against ND with the vector vaccine rHVT-F.
The objective of the use of the vector vaccine rHVT-F was twofold: a) to assess the vector vaccine’s field safety and induced seroconversion and, b) to control ND seroconversion and NDV circulation in some of the company’s integrated farms due to lateral contamination of those farms with the NDV vaccine strain HB1 and/or La Sota (5) most probably originated from either/both broiler breeder or/and commercial layer farms located in the same states. No overt clinical disease (high mortality, neurological signs) and none ND typical organ lesions (e.g., hemorrhages and necrosis in the gastro-intestinal and reproductive tracts, thymus and Bursa of Fabricius) were ever expressed by these NDV vaccine strains laterally-infected non-ND vaccinated broiler flocks. However, some slight increase in late grow out period mortality by bacterial secondary infections and a slight increase in carcass condemnation also by bacterial lesions (airsacculitis, septicemia) were correlated with those flocks seroconverting to ND. This is a typical clinical situation seen in broiler flocks vaccinated with live ND vaccines (HB1/La Sota).
In both, Companies 1 and 2 above, all broiler flocks had absolutely the same vaccination program (except for Newcastle), management procedures, nutrition levels, type of houses and disease challenges in general.
Sampling for laboratory analysis
Blood sampling for Elisa serology ((Idexx® NDV Ab Test) was carried out in 10 different broiler farms at different broiler ages during the grow put period at Company 1 and in 8 broilers farms at the slaughter age in Company 2.
Productivity data
When available, some key productivity parameters (daily weight gain, slaughter weight, feed conversion, final mortality and European productivity index) were per-flock collected and statistically analyzed at both companies for the different experimental groups (vector rHVT-F vaccinated broilers and not ND vaccinated broilers).
Statistical analysis
When appropriate, clinical, productivity and laboratory results were statistically analyzed by completely random analysis of variance and means compared by Tukey HSD All-Pairwise test at p<0.05 level. (Statistix 9.0 software – www.statistix.com)
Results
Company 1
Figure 1 depicts the ND Elisa titers throughout the grow out period for broilers not vaccinated against ND and broilers vaccinated with a vector rHVT-F vaccine. At all sampling ages the titers were not different between both experimental groups.
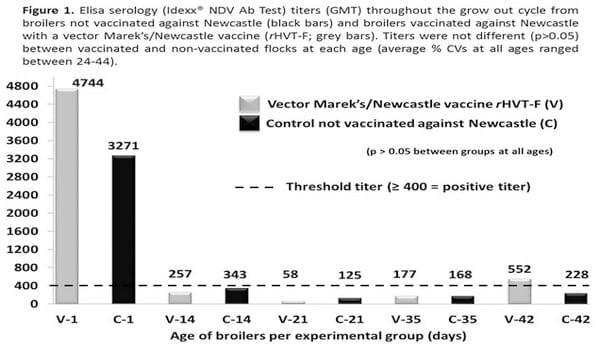
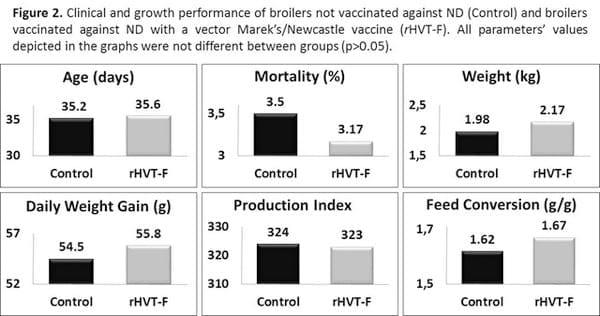
In addition, the clinical and growth performance data presented in Figure 2, equally indicate no difference between ND vaccinated and not vaccinated broilers.
Company 2
In Figure 3, ND Elisa titers for eight different broilers farms (integrated small farms) are shown subsequently for broilers not vaccinated against ND (previous flock laterally infected with the NDV live vaccine strain HB1), and the immediately next flock vaccinated with the vector rHVT-F vaccine via in ovo application. Vaccinated broilers presented very lower ND Elisa titers at slaughter age, in fact, in most of the farms the flocks were either negative or barely positive on the ND Elisa.
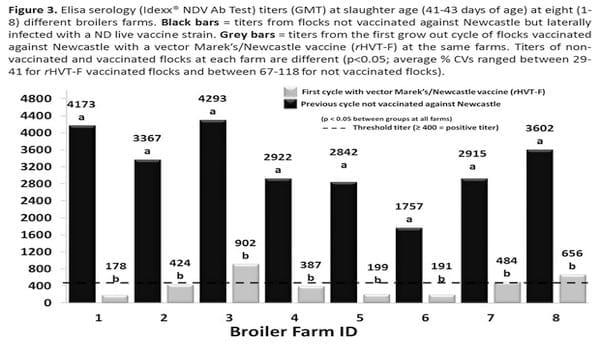
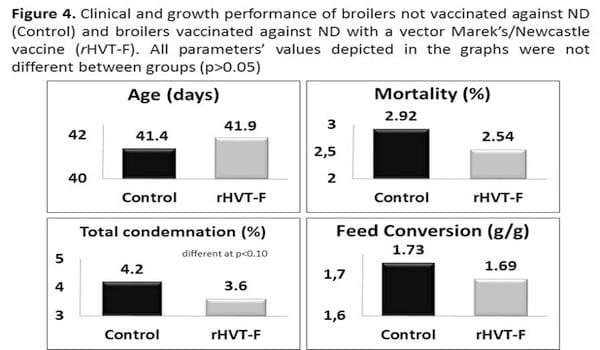
A tendency for a slightly numerically better (not different at p<0.05) clinical and productivity performance was detected in flocks vaccinated with the vector rHVT-F (Figure 4) when compared with immediately previous flocks not ND vaccinated and laterally contaminated by the live NDV vaccine strain HB1.The total % of carcass condemnation rate at slaughter was statistically lower (at p<0.10) for broilers vaccinated with the rHVT-F vaccine.
Discussion And Conclusions
The effectiveness of the rHVT-F ND vaccine in protecting broilers and commercial layers against experimental and field challenges from very virulent velogenic NDV has been recently and clearly confirmed in several instances (2,3,6,7,8,9). However, due to some specific ND epidemiological status and/or international market goals, some countries choose not to vaccinate against ND or to selectively vaccinate certain types of industrial birds only. This is the case of Brazil where ND vaccination is optional and many small, medium and large broiler integrator companies, located in southeastern and southern regions, have chosen not to vaccinate commercial broilers (only broiler breeders and commercial layers) since more than 20 years ago. The reasons for such decision were: a) avoid economic losses induced by post vaccination reaction induced by live commercial vaccines and, b) be able to effectively monitor any eventual NDV circulation in the companies through very a simple and cheap slaughter-age Elisa or HI serology.
Serology, clinical and growth performance of broilers vaccinated with the rHVT-F vaccine in companies 1 and 2, clearly indicate that it was able to effectively control NDV vaccine strain circulating in broilers flocks (Company 2) as well as inducing very low to negative antibody and very uniform titers as measured by a conventional ND Elisa (Companies 1 and 2).
In conclusion, the rHVT-F ND vaccine tested in this field trial was able to completely control NDV circulation in broilers while still allowing the production systems to maintain a cheap, quick and affective tool for monitoring NDV infections in their broiler flocks (slaughter-age serology). Therefore, the rHVT-F vaccine has provided de companies with an effective immunological tool that will protect the flocks against ND without interfering with the NDV-free status of the region.
References
1. Armour, N.K. and M. García. Current and future applications of viral-vectored recombinant vaccines in poultry. The Poultry Informed Professional, issue 134, July/August 2014.
2. Esaki, M., A. Godoy, J.K. Rosenberger, S.C. Rosenberger, Y. Gardin, A, Yasuda and K.M. Dorsey. Protection and Antibody Response Caused by Turkey Herpesvirus Vector Newcastle Disease Vaccine. Avian Disease 57:750-755, 2013
3. Merino, R., D. Dueñas, A. Soto, H. Flores, M. Lechuga. Evaluation of recombinant HVT NDV vaccines in laying hens experimentally challenged with the Mexican NDV virulent Chimalhuacan strain. Proceedings International Poultry Scientific Forum - Concurrent Meeting of the Southern Poultry Science Society 34th Annual Meeting / The Southern Conference on Avian Diseases 54th Annual Meeting - January 28-29, 2013 - Georgia World Congress Center, Atlanta, Georgia, USA, poster 132, 2013.
4. Miller, PJ & G. Koch. Newcastle Disease. In: Diseases of Poultry (DE Swayne, JG Glisson, LR McDougald, LK Nolan, DL Suarez & V Nair, editors) 13th edition, 2013, Wiley-Blackwell – John Wiley and Sons Inc., Ames, Iowa, USA – 2013
5. Orsi, M.A. Biological, molecular and immunological characterization and thermo-stability of vaccine strains and field isolates of Newcastle Disease virus from industrial and migratory poultry in Brazil. DSc Thesis, School of Medical Sciences, State University of Campinas (UNICAMP), Campinas, SP, Brazil, 179 pages, 2010
6. Palya, V., T, Tátar-Kis, T. Mató, B. Felföldi, E. Kovács and Y. Gardin. Onset and long-term duration of immunity provided by a single vaccination with a turkey herpesvirus vector ND vaccine in commercial layers. Veterinary Immunology and Immunopathology 158:105–115, 2014.
7. Palya, V., T. Tátar-Kis, T. Mató, B. Felföldi, E. Kovács and Y. Gardin. High level of protection achieved by vaccination with a recombinant HVT-NDV vector vaccine against different genotypes of Newcastle disease virus present in Latin America. Proceedings 63rd Western Poultry Disease Conference/XXXIX ANECA, April 1-5 2014, Puerto Vallarta, Jalisco, Mexico, pages 268-271, 2014.
8. Rauw, F., Y. Gardin, V. Palya, T. van den Berg and B. Lambrecht. The combination of attenuated Newcastle disease (ND) vaccine with rHVT-ND vaccine at 1-day old is more protective against ND virus challenge than when combined with inactivated ND vaccine. Avian Pathology, 43:26-36, 2014
9. Sesti, L., C. Kneipp, R. Paranhos, P. Paulet, and C. Cazaban. Field safety and efficacy of a vector Marek’s/Newcastle disease vaccine (rHVT – NDV) as assessed by clinical and productive performance in a large population of commercial broilers. Proceedings 62nd Western Poultry Disease Conference, March 25-27, 2013, Sacramento, CA, USA, pages 19-22, 2013.
Related topics:
Authors:
Ceva Animal Health
Recommend
Comment
Share
5 de septiembre de 2016
BONJOUR
NORMALEMENT AVEC LE VECTORISE ONT AURA DES TITRES ELEVES POUR PROTEGER LES POULET D'UN PHENOTYPE 7 EN ALGERIE TRES VIRULENT PAR CE VACCINS ONT POURA PAS NOUS PROTEGER SELON VOUS LE HBI QUI UNE SOUCHE TRES FAIBLE A MIEUX TITRE QUE VECTORMUNE F QUE PONSER VOUS MERCI
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.









.jpg&w=3840&q=75)