Photostimulation effects on reproductive activities of domestic birds
Published: April 4, 2014
By: Prof. Israel Rozenboim, MobarkeyN.,Avital-Cohen N.1,Kashash- Hanin Y1.Heiblum R.(Hebrew University of Jerusalem),Chaiseha Y2 (Suranaree University of Technology)and El Halawani M.E3 (University of Minnesota)
The role of light in biological activities associated with avian growth and reproduction is very well known. Light quality can be defined by three criteria: 1) daily pattern of lightdark exposure; photoperiod, 2) light intensity (brightness), and 3) spectral composition (Andrews and Zimmerman, 1990).
The role of light in avian reproduction
Photoperiod: in birds from subtropical and temperate latitudes, the gradual or abrupt increase in day length (long photoperiod; LP) initiates gonad recrudescence and egg laying. Conversely, reduction in day length (short photoperiod; SP) delays the onset of sexual maturity and may even terminate terminates egg laying activity in birds (Benoit, 1964 and Woodard et al., 1969).
Light intensity: plays an important role in rearing birds, mainly because birds need a certain light intensity in order to be photostimulated (North et al.,1990). Previous study conducted in our lab found a close relationship between light intensity and light spectra of laying hens. We found that by using optimal light spectra, light intensity can be decreased sharply, resulting in a significant decrease in the feed intake of the birds. This method of lighting laying hens can be highly beneficial for farmers, as savings in rearing costs were observed. Furthermore; we found that rearing birds under 0.01W/m2 at bird head level, significantly reduced feed intake, regardless of light spectra (Rozenboim, et al., 1998). Boshouwers and Nicaise (1987) had examined the effect of light intensity on energy expenditure of laying hens; a decrease in light intensity from 120 to 1 lux was found to reduce the total energy expenditure by 18%. This phenomenon was due to a reduction in activity-related energy expenditure in birds reared under low light intensity. Furthermore, low light intensity was found to reduce both heat production and core body temperature, probably due to a reduction in physical activity (Li et al.,1992). On the contrary, Charles (1984) suggested that light intensity does not play an important role in feed intake of laying hens.
Light spectra: The chicken eye, in similarity to the human eye, is capable of seeing in a narrow part of the light spectrum (380 to 760nm). Apart from the eyes, birds are equipped with active extraretinal photoreceptors (ERPRs), located in several parts of the brain, and are involved in transduction of photostimulation. Photostimulation, as affected by different wavelengths, has been discussed previously regarding chickens (Harrison et al., 1970), turkeys (Scott and Payne, 1937), sparrows (Ringoen, 1942), ducks (Benoit, 1964), and quails (Phogat et al., 1985). In general, red light stimulates egg production efficiently, whereas green or blue light have little or no effect. In commercial layers, during the first and second laying season, total egg production was significantly influenced by light color, with the largest amount of eggs produced in the red light-treated group. Furthermore, eggs laid under blue or green light were consistently larger than those laid under the red light (Pyrzak et al., 1987).
Photoreception affects sexual activities: Many avian species are photoperiodic and respond to long photoperiods by activation of the reproductive axis. The neuroendocrine response to photostimulation is reflected by a significant release of gonadotropin-releasing hormone I (GnRH-I) (Dunn and Sharp, 1999) followed by pituitary secretion of gonadotropins (Lewis et al., 2005, Lewis et al., 1998) resulting in gonad recrudescence (Sharp, 2005).
Three major sites have been shown to contain photoreceptors; the eyes (retina), the pineal gland and the deep brain tissue (ERPRs) (Foster and Soni, 1998; and Rathinam and Kuenzel, 2005). Photic cues that regulate the timing of seasonal reproductive cyclicity in birds are detected by extra-retinal photoreceptor (Kuenzel et al., 1993 and Malik et al., 2004). In contrary to the pivotal role of the pineal in mammals, enucleation and/or pinealectomy in birds caused no change in the pattern of seasonal changes of gonadal growth and secretion of luteinizing hormone (LH) (Wilson, 1991). However, covering the head so that the light cannot penetrate the skull eliminated the photoperiodic responses (Menaker et al., 1970).
Photoreceptors were suggested to be involved in the detection of daily or seasonal changes of photoperiod (Etches, 1996). All photoreceptor cells contain a large protein; an opsin, covalently bound to the aldehyde moiety of vitamin A; the chromophore (Bownds, 1967). When the chromophore absorbs a photon, the molecule isomerizes from 11-cis to all-trans configuration (Hart, 2001). This in turn leads to a conformational change in the opsin that triggers its enzymatic activity and initiates a biochemical cascade that causes a change in the rate of neurotransmitter release from the photoreceptor (Applebury and Hargrave 1986).
It has been demonstrated that domestic fowls subjected to gonad stimulating photoperiod, responded to the longer wavelengths of the spectrum (Harrison et al., 1970). The sensitivity of the bird to long wave radiation (630-780 nm) is a result of deep tissue penetration (hypothalamic ERPRs) stimulating reproductive axis (Benoit and Assenmacher, 1966 and Menaker and Underwood, 1976). In contrast, activation of retinal photoreceptors appears to cause an inhibitory effect on reproduction (Homma et al., 1972, Siopes and Wilson, 1980a,b). The response to visible radiation is probably mediated by the greenyellow bands of the light spectrum (545-575 nm), where the avian retina is in a relative peak sensitivity (Prescott and Wathes, 1999a,b).
The effects of photostimulation on hypothalamicgonadal axis are well characterized, whereas the mechanisms that transduce relevant photic information to neuroendocrine effector neurons are not well established (Cho et al., 1998; Dawson and Goldsmith, 1997 and Peczely and Kovacs, 2000). The link between ERPRs and the reproductive axis remains an open question. Saldanha et al., (2001) reported that brain photoreceptors communicate directly with GnRH-neurons, to stimulate the reproductive axis. Additional link could be vasoactive intestinal peptide (VIP) cells, which co-localize with all opsin-expressing cells in birds (Moore et al., 1978; Opel and Proudman, 1988). The VIP within the opsin system has the potential to regulate reproductive function via synaptic interactions all along the trajectory of its axons through the lateral septum (LS) and hypothalamus.
Two studies were conducted in order to investigate the relationship between retinal and extra retinal photoreception in reproductive activities of domestic birds. The first was conducted in by Prof M.E El Halawani and colleagues on turkey hens, and the second was conducted in our laboratory, on broiler breeder hens (Mobarkey et al., 2010). The objective of both studies was to provide further understanding of the interplay between light spectra photoreception and its association to the modulation of the hypothalamic-pituitary-gonadal axis in both turkeys and broiler breeder hens.
Three light treatments given in the experiments were white light (control), and 2 parallel light circuits, installed in each of the two light-controlled rooms in the turkey experiment, or in the top of the cages in the broiler breeder experiment. The first circuit (red) had peak emission in the 650-725 nm range and the second circuit (green) had peak emission between 500 to 575 nm. Before photostimulation, birds were kept under non-photostimulatory conditions i.e. 6 hr of light using both the red and the green light circuits. Hens were then photostimulated by increasing the day length to 16 hr of either the red light circuit (red group) or the green light circuit (green group). The second light circuit in each room remained at 6 hr.
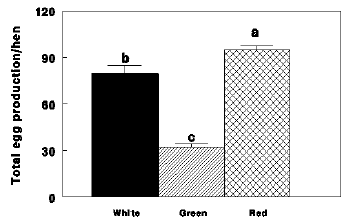
In the turkey hen experiment peak egg production did not differ between the control white and red light treatment groups in weeks 5, 6, and 7 of photostimulation. Thereafter, the decline in egg production in the control birds was grater than that of the red group, and the differences between the two groups were significant in weeks 9, 14, 15, and 22 of photostimulation (P<0.05, Figs. 1 and 2). The green light group exhibited a different egg laying pattern than that of the control and red light groups following photostimulation, and their egg production remained consistently low throughout the experimental period. The overall mean egg production during the 27 weeks experimental period was significantly greater in the red light birds compared with white light control or the green light groups (Fig. 2)
Figure 1 - Weekly egg production of Nicholas turkey hens reared under 16 hr of green light and 6 hr of red light photoperiod (Green), or 16 hr of red light and 6 hr of green light photoperiod (Red), or reared under 16 hr of full spectra of light (White). Data presents mean ! S.E. *significant difference from Green until the end of the experiment.**Significant difference between Red and White.
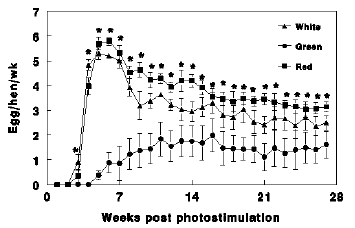
Figure 2 - Total egg production of Nicholas turkey hens reared under 16 hr of white light (White), 16 hr of green light combined with 6 hr of red light (Green) and 16 hr of red light combined with 6 hr of green light (Red). Data presents mean ! S.E. a, b, c, - bars marked with different letters are significantly different (P<0.05).
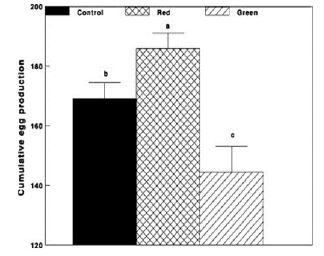
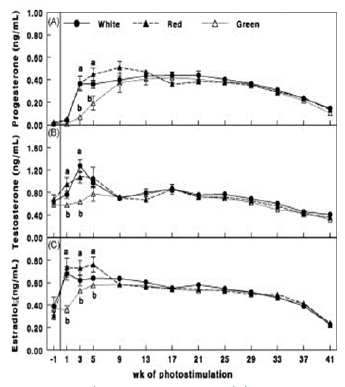
Similar results were found in broiler breeder hens. Green group had lesser egg production (Figs. 3 and 4), lesser plasma concentrations of ovarian steroids (Fig. 5).
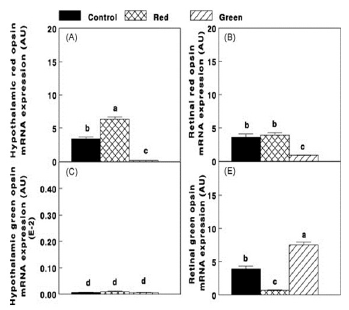
Photostimulation of ERPRs (Red group) caused an elevation (P≤0.05) in hypothalamic red opsin mRNA expression (Fig. 6A). Red opsin expression was also observed in the retina (Fig. 6B). On the other hand selective photostimulation of retinal photoreceptors (Green group) caused an elevation (P≤0.05) in retinal green opsin (P≤0.05, Fig. 6C), whereas the expression of green opsin in the hypothalamus was very low in all groups (Fig. 6D).
Figure 3 - Egg production (%) of Cobb broiler breeder hens of the Control group (29 lux); Red group, photostimulated with red light (29 lux) combined with non-photostimulatory green light (27.5 lux); and Green group, photostimulated with green light combined with non-photostimulatory red light. Data are presented as mean ± standard error of the mean (n = 45). Values with different letters are significantly different (P ≤ 0.05).
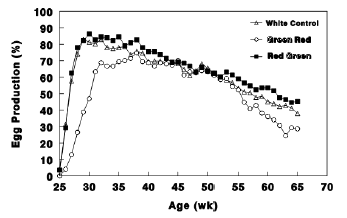
Figure 4 - Cumulative egg production of Cobb broiler breeder hens of the Control group (29 lux); Red group, photostimulated with red light (29 lux) combined with non-photostimulatory green light (27.5 lux); and Green group, photostimulated with green light combined with non-photostimulatory red light. Data are presented as mean ± standard error of the mean (n = 45). Values with different letters are significantly different (P ≤ 0.05).
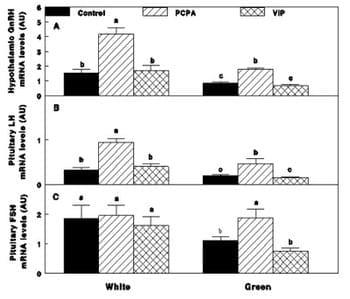
Photostimulation of ERPRs (Red group) caused an elevation (P≤0.05) in GnRH-I mRNA expression in the hypothalamus, whereas selective photostimulation of retinal photoreceptors (Green group) had no effect on GnRH-I mRNA expression (Fig. 7A). A decrease (P≤0.05) in LH mRNA expression (Fig. 7C) was detected in the Green group, whereas the decrease in FSH mRNA expression was not significant (Fig. 7B).
Figure 5 - Plasma progesterone (A), testosterone (B) and estradiol (C) concentrations of Cobb broiler breeder hens of the Control group (29 lux); Red group, photostimulated with red light (29 lux) combined with non-photostimulatory green light (27.5 lux); and Green group, photostimulated with green light combined with non-photostimulatory red light. Plasma steroid concentrations were determined by enzyme-linked immunosorbent assay. Data are presented as mean ± standard error of the mean (n = 45). Values with different letters are significantly different (P ≤ 0.05).
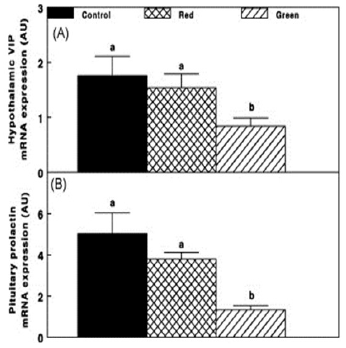
Retinal and extra-retinal photostimulation also affected the lactotrophic axis. Hypothalamic VIP mRNA expression was reduced in retinal photostimulated hens (Green group, Fig. 8A) and was positively correlated with decreased prolactin mRNA expression (p ≤0.05; Fig. 8B).
These results, taken together, support previous findings that the eyes are not necessary for photic determination of reproductive stimulation. Moreover, it seems that the eyes have an inhibitory effect on reproduction, in accordance with the fact that orbital enucletion increased egg production in chickens (Harrison, 1972). In the present study, selective photostimulation with green light was accompanied with greater retinal green opsin mRNA expression. Conversely, very low hypothalamic green opsin gene expression was detected, since the ability of green light to penetrate the tissue is poor (Wan,et al., 1981). However, long wave length radiation (red band of the spectrum) has the ability to penetrate the tissue (Benoit, 1978) and therefore, we detected greater red opsin gene expression in the hypothalamus which has maximum absorbance at 630 nm (Benoit, 1978).
Figure 6 - Hypothalamic and retinal red opsin mRNA expression (A and B, respectively) and hypothalamic and retinal green opsin (C and D, respectively) of the Control group (29 lux); Red group, photostimulated with red light (29 lux) combined with nonphotostimulatory green light (27.5 lux); and Green group, photostimulated with green light combined with non-photostimulatory red light. Expressions of red and green opsin were determined by real-time polymerase chain reaction. Abbreviations: A.U., arbitrary units; E-2, results×10-2. Data are presented as mean ± S.E. of the mean (n = 5). Values with different letters are significantly different (P ≤ 0.05).
The role of retinal photoreceptors in reproductive activities of broiler breeder hens
The mechanism by which the eyes inhibit reproduction is unknown. The first candidate that should be investigated in relation to the inhibitory role of the eyes in reproduction is serotonin, since high levels of serotonin, which is synthesized in the retina (Homma, et al., 1972 and Opel and Proudman,1988) and in the hypothalamus (Redburn, 1984) during the day (Homma, et al., 1972; Opel and Proudman,1988 and Suzuki et al., 1977) have been reported to inhibit avian reproduction (Braganza and Wilson,1978; Suzuki et al., 1981 and El Halawani et al.,1983). The second candidate that should be investigated is vasoactive intestinal peptide (VIP), since its synthesis and release, in birds, is under the control of serotonin (Hall et al., 1986; Li and Pelletier, 1995; El Halawani et al.,1995; Moore et al.,1978 and Palkovits, et al.,1977) and consequently, VIP levels increase during the day and decrease during the night (Shinohara et al., 1993). Moreover, VIP is a prolactin-releasing factor (El Halawani et al.,1990) and prolactin at high concentrations inhibits reproduction (Youngren, et al., 1991).
Figure 7 - Chicken GnRH, LH and FSH mRNA expression (A, B, C) of the Control group (29 lux); Red group, photostimulated with red light (29 lux) combined with non-photostimulatory green light (27.5 lux); and Green group, photostimulated with green light combined with non-photostimulatory red light. Expressions of gonadotropin-releasing hormone (GnRH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH) were determined by semi quantitative PCR. Abbreviation: A.U., arbitrary units. Data are presented as mean ± standard error of the mean (n = 4). Values with different letters are significantly different (P ≤ 0.05).
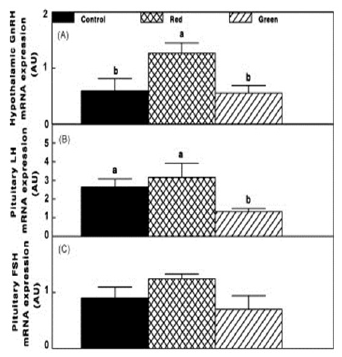
Figure 8 - Chicken hypothalamic vasoactive intestinal peptide (VIP) and pituitary prolactin mRNA expression of the Control group (29 lux); Red group, photostimulated with red light (29 lux) combined with non-photostimulatory green light (27.5 lux); and Green group, photostimulated with green light combined with non-photostimulatory red light. Abbreviation: A.U., arbitrary units. Data are presented as mean ± standard error of the mean (n = 4). Values with different letters are significantly different (P ≤ 0.05).
We studied the mechanism underlying the decrease in reproductive performances following retinal photostimulation (green light) by investigating the role of VIP and serotonin. Parachlorophenylalanine (PCPA) treatment, an inhibitor of serotonin (5-HT) biosynthesis, increased reproductive performances (Fig. 9) and mRNA expression of GnRH-I, LH-" and FSH-b" (Fig. 10) of the Green group up to levels which did not differ from the white control group.
Serotonin may suppress the reproductive axis activity via two pathways: A. Directly through serotonin receptor type 2, 5-HT2 (Halawani and Burke, 1976) which suppresses GnRH-I synthesis (El Halawani et al., 1983) and LH secretion (Halawani and Burke, 1976). Serotonin decreases GnRH and LH release in female rats after sexual maturity (Arias et al., 1990 and Moguilevsky and Wuttke, 2001). We suggest that a similar mechanism exists also in birds, since PCPA treatment in the present study of mature broiler breeder chickens, increased GnRH-I, LH-b" and FSH-b" mRNA expression in agreement with the finding in female rats after sexual maturity. B. Indirectly, involving different possible modulators, such as VIP, as prolactin -releasing hormone, (El Halawani et al.,1995) that, as mentioned before, its synthesis is controlled by serotonin (El Halawani et al.,1988; Hargis and Burke, 1984; Macnamee and Sharp, 1989). In the present study, immunization against VIP lowered prolactin mRNA expression and its plasma levels without any effect on reproductive performances. A possible explanation for this is that VIP neutralization may have decreased the direct stimulatory effect of VIP. VIP expression was demonstrated in the embryo chicken retina (Teruyama and Beck, 2001) and VIP terminals have a direct synaptic contact with GnRH cells (Van der Beek et al., 1994; Van der Beek et al., 1993 and Prada Oliveira et al., 2003). Therefore, it has been suggested that VIP may be a transducer of stimulatory photic cues to the GnRH system in birds (Saldanha et al., 1994; Saldanha et al., 2001) and that VIP neutralization should decrease reproductive axis activity. On the other hand, although PCPA treatment in the present study significantly lowered VIP mRNA expression, an elevation in reproductive performances was observed. Taken together, our study indicates that serotonin, and not VIP, is involved in the reproductive decline associated with selective retinal photostimulation.
Figure 9 - Egg production (%) of Cobb broiler breeder hens reared under photostimulatory white light (29 lx)-White or green light (27.5 lx) combined with non photostimulatory red light-Green. Hens were treated with PCPA (White-PCPA, Green-PCPA), actively immunized against VIP (White-VIP, Green- VIP) or were untreated (White-Control, Green- Control). Single arrow indicates PCPA treatment and double arrows indicate VIP immunization and PCPA treatment. Data are presented as mean ± S.E.M (n = 15). Values with different letters are significantly different (P < 0.05).
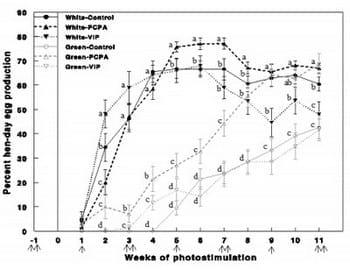
Figure 10 - GnRH-I, LH-b! and FSH-b! mRNA expression (A, B, C), determined by semi-quantitative PCR, of Cobb broiler breeder hens reared under photostimulatory white light (29 lx)-White or green light (27.5 lx) combined with non photostimulatory red light-Green. Hens were treated with PCPA (White-PCPA, Green-PCPA), actively immunized against VIP (White-VIP, Green-VIP) or were untreated (White-Control, Green-Control). Data are presented as mean ± S.E.M (n = 4). Values with different letters are significantly different (P < 0.05).
New evidence for epigenetic manipulation in retinal opsin mRNA level
Fertilized avian eggs are usually incubated in darkness. Previous studies have shown that exposing avian eggs (e.g., broilers, White Leghorn chickens, turkeys and quails), to white or green light during incubation, increases the embryo body weight and accelerates hatching (Shultz, et al., 1962; Siegel, et al., 1969; Cooper, 1972; Walter and Voitle, 1972; Coleman and Mcnabb, 1975; Guatpande et al., 1995; and Shsfey and Al-Mohsen, 2002). Intermittent light regime (15 min. on and 15 min. off) enabled to study the definite effects of green light illumination on body and muscle growth in broilers and turkeys (Rozenboim et al., 2003, 2004). Combinations of light illumination in pre- and posthatch broilers (dark vs. green light during embryonic period followed by white or green light post-hatch) revealed that the best effect on the development and growth of the chicks, is achieved when green light stimulus is provided during incubation. Taking together with recent study presented in this paper (Mobarkey, et al., 2010), that showed that in response to green light photostimulation there was a significantly higher expression of the green opsin receptor gene, and a lower red opsin receptor gene expression in the retina, we suggested that green light, which stimulates mainly the retinal photoreceptors, apparently causes suppressed reproductive performances in adult chickens. The objective of our current study was to examine the pattern of expression of the cone photoreceptors (i.e. green and red opsins) in the retina of the developing chicken embryo during incubation. Furthermore, we tested the effect of incubation under monochromatic green or red light manipulation on the expression of the green and red opsin genes.
Findings yielded from this study, so far, show that opsin RNA transcription is first evident by Real-Time PCR technique at E14 (The 14th day of incubation). This is consistent with the findings of Bruhn and Cepko (1996) who used the in situ hybridization technique.
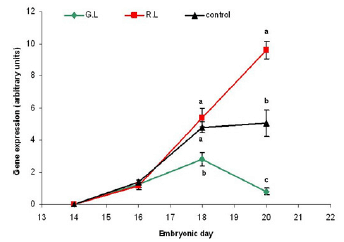
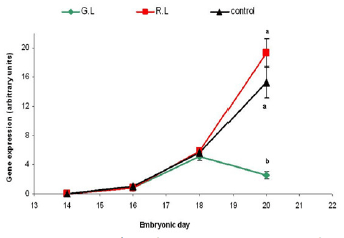
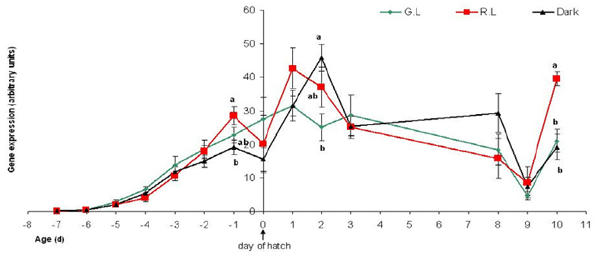
In Addition we found that green light during incubation suppresses the green and red opsin receptors gene expression in the last three days before hatching, while red light enhances their expression (Figures 11-12).
Figure 11 - Green opsin mRNA gene expression in embryo retina (arbitrary units). Embryos were incubated under monochromatic light throughout the embryonic period. G.L= Green Light; R.L= Red Light; Control= dark. n=10 in each treatment group, on each sampling point (embryonic day). Results are presented as MEAN±SEM. Different letters represent a statistically significant difference (p<0.05).
In an extended experiment, we tested embryos from E0, through hatch and up to 10-day old chicks, which were incubated under red/green monochromatic light in the hatchery and were then reared in a room with regular fluorescent light, much the same as the lighting provided in commercial chicken pans. Although not statistically significant throughout the experimental period, results from the latter experiment show a lasting effect of the down regulation of the green and red opsins in response to incubation under monochromatic green lighting, which lasted up to 9 days post hatch (Figures 13-14). These results lead us to believe that there might be an epigenetic effect of green and red light wavelengths on the embryo’s genome that may even last to adulthood. Providing lighting inside the hatchery is relatively easy to implement and not too expensive. In light of this, we wish to further investigate the potential possibilities for manipulating the expression of retinal and extra retinal photoreceptors using light of different wavelengths, and in different intensities.
Figure 12 - Red opsin mRNA gene expression in embryo retina (arbitrary units). Embryos were incubated under monochromatic light throughout the embryonic period. G.L= Green Light; R.L= Red Light; Control= dark. n=10 in each treatment group, on each sampling point (embryonic day). Results are presented as MEAN±SEM. Different letters represent a statistically significant difference (p<0.05).
Nowadays we are working on analyzing the expression of the red and green opsins in the brains of the light-treated embryos and chicks, in order to determine the effect of light during incubation on the ERPRs as well.
Thorough and extended research is required in order to reveal the molecular mechanism by which the light affects the chicken’s genome, and the indirect way it influences its reproductive performances.
Figure 13 - Green opsin mRNA gene expression in the eye retina (arbitrary units), of embryos on days E14-E20 of incubation (represented on the graph as days -7 to -1 before hatch), and chicks from day of hatch (“0”) until 10 days of age. The embryos were incubated under Green/Red monochromatic light throughout the incubation period. Incubation in the dark was used as control. G.L= Green Light; R.L= Red Light; Control= dark. n=7 in each treatment group, on each sampling point. Results are presented as MEAN±SEM. Different letters represent a statistically significant difference (p<0.05).
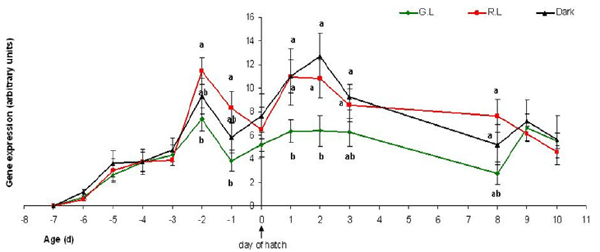
Figure 14 - Red opsin mRNA gene expression in the eye retina (arbitrary units), of embryos on days E14-E20 of incubation (represented on the graph as days -7 to -1 before hatch), and chicks from day of hatch (“0”) until 10 days of age. The embryos were incubated under Green/Red monochromatic light throughout the incubation period. Incubation in the dark was used as control. G.L= Green Light; R.L= Red Light; Control= dark. n=7 in each treatment group, on each sampling point. Results are presented as MEAN±SEM. Different letters represent a statistically significant difference (p<0.05).
References
ANDREWS D.K. and ZIMMERMAN N.G. (1990) A comparison of energy efficient house lighting source and photoperiods. Poultry Science 69:1471-1479.
APPLEBURY M.L. and HARGRAVE P.A. (1986) Molecular biology of the visual pigments. Vision Research 26:1881-1895.
ARIAS P., SZWARCFARB B., DE RONDINA D.C., CARBONE S., SVERDLIK R. and MOGUILEVSKY J.A. (1990) In vivo and in vitro studies on the effect of the serotoninergic system on luteinizing hormone and luteinizing hormone-releasing hormone secretion in prepubertal and peripubertal female rats. Brain Research 523:57- 61.
BENOIT J. (1964) The role of the eye and the hypothalamus in the photostimulation of gonads in the duck. Annals of the New York Academy of Science 111:204-216.
BENOIT J. and ASSENMACHER I. (1966) Research on the photosensitivity of superficial and deep neural receptors in gonad stimulation by visible radiation in the immature Pekin duck [in French]. Comptes Rendus Hebdomadaires des Seances de l Academie des Sciences. D: Sciences Naturelles 262:2750-2752.
BENOIT J.M. (1978) Chronobiologic study in the domestic duck. II. Physiological mechanism of the chronobiologic action of visible light on the gonads of the male duck. Chronobiologia 5:158-168.
BOSHOUWERS F.M. and NICAISE E. (1987) Physical activity and energy expenditure of laying hens as affected by light intensity. British Poultry Science 28:155-63.
BOWNDS D. (1967) Site of attachment of retinal in rhodopsin. Nature 216:1178-1181.
BRAGANZA A. and WILSON W.O. (1978) Elevated temperature effects on catecholamines and serotonin in brains of male Japanese quail. Journal of Applied Physiology 45:705-708.
BRUHN S.L. and CEPKO C.L. (1996) Development of the pattern of photoreceptors in the chick retina. The Journal of Neuroscience 16:1430-1439.
CHARLES D.R. (1984) A model of egg production. British Poultry Science 25:309-321.
CHO N., HARADA M., IMAEDA T., IMADA T., MATSUMOTO H., HAYASE Y., SASAKI S., FURUYA S., SUZUKI N., OKUBO S., OGI K., ENDO S., ONDA H. and FUJINO M. (1998) Discovery of a novel, potent and orally active nonpeptide antagonist of the human luteinizing hormone-releasing hormone (LHRH) receptor. Journal of Medicinal Chemistry 41:4190- 4195.
COLEMAN M.A. and MCNABB R.A. (1975) Photoacceleration of embryonic development in depigmented Japanese quail eggs. Poultry Science 54:1849-1855.
COOPER J.B. (1972) Effect of light during incubation on hatchability of turkey eggs. Poultry Science 51:1105- 1108.
DAWSON A. and GOLDSMITH A.R. (1997) Changes in gonadotrophin-releasing hormone (GnRH-I) in the preoptic area and median eminence of starlings (Sturnus vulgaris) during the recovery of photosensitivity and during photostimulation. Journal of Reprodution and Fertility 111:1-6.
DUNN I.C. and SHARP P.J. (1999) Photo-induction of hypothalamic gonadotrophin releasing hormone-I mRNA in the domestic chicken: A role for oestrogen? Journal of Neuroendocrinology 11:371-375.
EL HALAWANI M. E., SILSBY J. L. and MAURO L.J. (1990). Vasoactive intestinal peptide is a hypothalamic prolactin-releasing neuropeptide in the turkey (Meleagris gallopavo). General and Comporative Endocrinology 78:66-73.
EL HALAWANI M.E., SILSBY J.L., FEHRER S.C. and BEHNKE E.J. (1983) Reinitiation of ovulatory cycles in incubating female turkeys by an inhibitor of serotonin synthesis, p-chlorophenylalanine. Biology of Reproduction 28: 221-228.
EL HALAWANI M.E., SILSBY J.L., ROZENBOIM I. and PITTS G.R. (1995) Increased egg production by active immunization against vasoactive intestinal peptide in the turkey (Meleagris gallopavo). Biology of Reproduction, 52:179-183.
EL HALAWANI M.E., YOUNGREN O.M., SILSBY J.L. and PHILLIPS R.E. (1988) Involvement of serotonin in prolactin release induced by electrical stimulation of the hypothalamus of the turkey (Meleagris gallopavo), General and Comporative Endocrinology, 72:323- 328.
ETCHES R.J. (1996) Reproduction in poultry, CAB international. UK. FOSTER R.G. and SONI B.G. (1998) Extraretinal photoreceptors and their regulation of temporal physiology. Reviews of Reproduction 3:145-150.
GUATPANDE A., GUATPANDE S. and KHAN, M.Z. (1995) Effect of different intensities of fluorescent light on the early development of chick embryos in ovo. Cellular and Molecular Biology Research 41:613-621.
HALAWANI M.E. and BURKE W.H. (1976) Brain monoamine metabolism of turkey hens in various stages of their reproductive life cycle. Biology of Reproduction 15:254-259.
HALL T.R., CHEUNG A. and HARVEY S. (1986) Serotoninergic inhibition of LH secretion in the domestic fowl. Journal of Endocrinology 110:239- 244.
HARGIS B.M. and BURKE W.H. (1984) Prolactin and luteinizing hormone levels of prelaying, laying and postlaying turkey hens following central administration of serotonin and peripheral administration of quipazine maleate. General and Comparative Endocrinology 55:12-19.
HARRISON P.C., LATSHOW D., CASEY J.M. and MCGINNIS J. (1970) Influence of decreased length of different spectral photoperiods on testis development of domestic fowl. Journal of Reproduction and Fertility 22:269-275.
HARRISON P.C. (1972) Extraretinal photocontrol of reproductive responses of leghorn hens to photoperiods of different length and spectrum. Poultry Science 51:2060-2064.
HART N.S. (2001) The visual ecology of avian photoreceptors. Progress in retinal and eye research 20:675-703.
HOMMA K., WILSON W. O. and SIOPES T.D. (1972) Eyes have a role in photoperiodic control of sexual activity of coturnix. Science 178:421-423.
KUENZEL W.J. (1993) The search for deep encephalic photoreceptors within the avian brain, using gonadal development as a primary indicator. Poultry Science 72:959-967.
LEWIS P.D., CIACCIARIELLO M., CICCONE N.A., SHARP P.J. and GOUS R.M. (2005) Lighting regimens and plasma LH and FSH in broiler breeders. British Poultry Science 46:349-353.
LEWIS P.D., PERRY G.C., MORRIS T.R., DOUTHWAITE J.A. and BENTLEY G.E. (1998) Effect of constant and of changing photoperiod on plasma LH and FSH concentrations and age at first egg in layer strains of domestic pullets. British Poultry Science 39:662-670.
LI S. and PELLETIER G. (1995) Involvement of serotonin in the regulation of GnRH gene expression in the male rat brain. Neuropeptides 29:21-25.
LI Y., ITO T., NISHIBORI M. and YAMAMOTO S. (1992) Effects of environmental temperature on heat production associated with food intake and on abdominal temperature in laying hens. British Poultry Science 33:113-22.
MACNAMEE M.C. and SHARP P.J. (1989) The functional activity of hypothalamic 5-hydroxytryptamine neurons in broody bantam hens. Journal of Endocrinology 120:125-134.
MALIK S., RANI S. and KUMAR V. (2004) Wavelength dependency of light-induced effects on photoperiodic clock in the migratory blackheaded bunting (Emberiza melanocephala). Chronobiology International 21:367- 384.
MENAKER M. and UNDERWOOD H. (1976) Extraretinal photoreception in birds. Photophysiology 23:299- 306.
MENAKER M., ROBERTS R., ELLIOTT J. and UNDERWOOD H. (1970) Extraretinal light perception in the sparrow. 3. The eyes do not participate in photoperiodic photoreception. Proceedings of the National Academy of Sciences of the United States of America 67:320- 325.
MOBARKEY N., AVITAL N., HEIBLUM R. and ROZENBOIM I. (2010) The role of retinal and extra-retinal photostimulation in reproductive activity in broiler breeder hens. Domestic Animal Endocrinology 38:235-243.
MOGUILEVSKY J.A. and WUTTKE W. (2001) Changes in the control of gonadotrophin secretion by neurotransmitters during sexual development in rats. Experimental and Clinical Endocrinology and Diabetes 109:188-195.
MOORE R.Y., HALARIS A.E. and JONES B.E. (1978) Serotonin neurons of the midbrain raphe: ascending projections. The journal of Comparative Neurology 180:417-438.
NORTH, M.O. and BELL D.D. (1990) Lighting management, Chicken Production Manual. Chapman and Hall, NY 10119.
OPEL H. and PROUDMAN J.A. (1988) Stimulation of prolactin release in turkeys by vasoactive intestinal peptide. Proceedings of the Society for the Experimental Biology and Medicine 187:455-460.
PALKOVITS M., SAAVEDRA J.M., JACOBOQITZ D.M., KIZER J.S., ZABORSZKY L. and BROWNSTEIN M.J. (1977) Serotonergic innervation of the forebrain: Effect of lesions on serotonin and tryptophan hydroxylase levels. Brain Research 130:121-134.
PECZELY P. and KOVACS K.J. (2000) Photostimulation affects gonadotropin-releasing hormone immunoreactivity and activates a distinct neuron population in the hypothalamus of the mallard. Neuroscience Letters 290:205-208.
PHOGAT S.B., AGGARWAL C.K. and CHOPRA S.K. (1985) Effect of red and green lights on growth of quail. Indian. Poultry Science 20:126-128.
PRADA OLIVEIRA J.A., VERASTEGUI ESCOLANO C., GOMEZ LUY C. and COLLANTES RUIZ J. (2003) Ontogenic attendance of neuropeptides in the embryo chicken retina. Histology and Histopathology 18:1013-1026.
PRESCOTT N.B. and WATHES C.M. (1999) Reflective properties of domestic fowl (Gallus g. domesticus), the fabric of their housing and the characteristics of the light environment in environmentally controlled poultry houses. British Poultry Science 40:185-193.
PRESCOTT N.B. and WATHES C.M. (1999) Spectral sensitivity of the domestic fowl (Gallus g. domesticus). British Poultry Science 40:332-339.
PYRZAK, R., SNAPIR N., GOODMAN G. and PEREK M. (1987) The effect of light wavelength on the production and quality of eggs of the domestic hen. Theriogenology 28:947-960.
RATHINAM T. and KUENZEL W.J. (2005) Attenuation of gonadal response to photostimulation following ablation of neurons in the lateral septal organ of chicks. Brain Research Bulletin 64:455-461.
REDBURN D.A. (1984) Serotonin systems in the inner and outer plexiform layers of the vertebrate retina. Federation Proceedings 43:2699-2703.
RINGOEN A.R. (1942) Effect of continuous green and red light illumination on gonadal response in English sparrow (Passar domesticus linnaeus). American Journal of Anatomy 71:99-112.
ROZENBOIM I., HUISINGA R., HALEVY O. and EL HALAWANI M.E. (2003) Effect of embryonic photostimulation on the posthatch growth of turkey poults. Poultry Science 82:1181-1187.
ROZENBOIM I., PIESTUN Y., MOBARKY N., BARAK M., HOYZMAN A. and HALEVY O. (2004) Monochromatic light stimuli during embryogenesis enhance embryo development and posthatch growth. Poultry Science 83:1413-1419.
ROZENBOIM I., ZILBERMAN E. and GVARYAHU G. (1998) New monochromatic light source for laying hens. Poultry Science 77:1695-1698.
SALDANHA C.J., LEAK R.K. and SILVER R. (1994) Detection and transduction of daylength in birds. Psychoneuroendocrinology 19:641-656.
SALDANHA C.J., SILVERMAN A.J. and SILVER R. (2001) Direct innervation of GnRH neurons by encephalic photoreceptors in birds. Journal of Biological Rhythms 16:39-49.
SCOTT H.M. and PAYNE L.F. (1937) Light in relation to the experimental modification of the breeding season of turkeys. Poultry Science 16:90-96.
SHARP P.J. (2005) Photoperiodic regulation of seasonal breeding in birds. Annals of the New York Academy Science 1040:189-199.
SHINOHARA K., TOMINAGA K., ISOBE Y. and INOUYE S.T. (1993) Photic regulation of peptides located in the ventrolateral subdivision of the suprachiasmatic nucleus of the rat: Daily variations of vasoactive intestinal polypeptide, gastrin-releasing peptide, and neuropeptide y. The Journal of Neuroscience 13:793- 800.
SHSFEY T.M. and AL-MOHSEN T.H. (2002) Embryonic growth, hatching time and hatchability performance of meat breeder eggs incubated under continuous green light. Asian-Australian Journal of Animal Science 15:1702-1707.
SHULTZ J.V., LAUBER J.K., KATO, M. and WILSON W. (1962) Influence of incandescent and coloured light on chicken embryos during incubation. Nature 196:594-595.
SIEGEL, P.B., ISAKSON, S.T., COLEMAN, F.N. and HUFFMAN B.J. (1969) Photoacceleration of development in chick embryo. Comparative Biochemistry and Physiology 28:753-758.
SIOPES, T.D. and WILSON, W.O. (1980) Participation of the eyes in the photosexual response of Japanese quail (Coturnix coturnix japonica). Biology of Reproduction 23:352-357.
SIOPES, T.D. and WILSON, W.O. (1980) Participation of the eyes in the photosexual (1980). Participation of the eyes in the photostimulation of chickens. Poultry Science 59:1122-1125.
SUZUKI, O., HATTORI, H. and ASANO, M. (1981) Identification and quantitation of 5-hydroxytryptamine in the retinae of cow, pig and chick by gas chromatography/mass spectrometry. The Japanese Journal of Pharmacology 31:470-473.
SUZUKI, O., NOGUCHI E., MIYAKE, S. and YAGI K. (1977) Occurrence of 5-hydroxytryptamine in chick retina. Experientia 33:927-928.
TERUYAMA, R. and BECK, M.M. (2001) Double immunocytochemistry of vasoactive intestinal peptide and cGnRH-I in male quail: Photoperiodic effects. Cell and Tissue Research 303:403-414.
VAN DER BEEK, E.M., VAN OUDHEUSDEN, H.J., BUIJS, RM, VAN DER DONK, H.A., VAN DEN HURK, R. and WIEGANT, V.M. (1994) Preferential induction of c-fos immunoreactivity in vasoactive intestinal polypeptideinnervated gonadotropin-releasing hormone neurons during a steroid-induced luteinizing hormone surge in the female rat. Endocrinology 134:2636-2644.
VAN DER BEEK E.M., WIEGANT V.M., VAN DER DONK H.A., VAN DEN HURK R. and BUIJS R.M. (1993) Lesions of the suprachiasmatic nucleus indicate the presence of a direct vasoactive intestinal polypeptidecontaining projection to gonadotrophin-releasing hormone neurons in the female rat. Journal of Neuroendocrinology, 5:137-144.
WALTER, J.H. and VOITLE, R.A. (1972) Effect of photoperiod during incubation on embryonic and post embryonic development of broilers. Poultry Science, 51:1122-1126.
WAN, S., PARRISH, J.A., ANDERSON, R.R. and MADDEN, M. (1981) Transmittance of nonionizing radiation in human tissues. Photochemistry and photobiology, 34: 679-681.
WILSON, F.E. (1991) Neither retinal nor pineal photoreceptors mediate photoperiodic control of seasonal reproduction in American tree sparrows (Spizella arborea). Journal of Experimental Zoology, 259:117-127.
WOODARD, A.E., MOORE, J.A. and WILSON, W.O. (1969) Effect of wave length of light on growth and reproduction in Japanese quail (Coturnix coturnix japonica). Poultry Science 48:118-123.
YOUNGREN, O.M., EL HALAWANI, M.E., SILSBY, J.L. and PHILLIPS, R.E. (1991) Intracranial prolactin perfusion induces incubation behavior in turkey hens. Biology of Reproduction 44:425-431.
Related topics
Authors:
The Hebrew University of Jerusalem
Recommend
Comment
Share
7 de abril de 2014
it is really informative article. I personally observed the photoperiodism in poultry specially in native breeds. thanks for information
Regards
Recommend
Reply
7 de abril de 2014
Hello Prof,
This is a very nice research work. I am interested to work in this area. And I really wish to have your contact details for further discussion. Thank you. Mathew
Recommend
Reply

Would you like to discuss another topic? Create a new post to engage with experts in the community.










.jpg&w=3840&q=75)