Infectious bronchitis: Reducing clinical signs in chickens
Published: August 10, 2015
By: Don McIntyre, Ph.D., PAS
Research and development at Diamond V over more than 70 years has led to product technologies proven to support the immune system, particularly in its response to respiratory challenges (Jensen et al., 2008 and Moyad et al., 2009). Building on this work, Embria Health Sciences, a division of Diamond V, developed the human supplement EpiCor®.
EpiCor demonstrated, through human clinical trials, a reduction in symptoms of colds, flu, allergies and hay fever (Moyad et al., 2009 and Moyad et al., 2010). The data have shown a reduction in the severity of symptoms and in length of time clinical signs persisted.
Similarly, published studies on chickens have reported an increase in antibody titers in Diamond V Original XPCTM-fed broilers vaccinated with Newcastle Disease virus (AlHomidan and Fahmy, 2007; Gao et al., 2008; Fathi et al., 2012). In all three published reports, scientists have documented elevated immune responses to Newcastle vaccination or challenge, indicating an improved level of protection in birds supplemented with Original XPC.
Given these reports, a study was designed to investigate the effects of feeding Original XPC with another respiratory disease, Infectious Bronchitis Virus (IBV). The following summary is from a study conducted at Auburn University College of Veterinary Medicine.
Experimental Design
Two separate trials were conducted using 200 Specific Pathogen Free (SPF) white leghorn chicks per trial (50 birds/treatment). In Trial 1, non-vaccinated birds were challenged with IBV. Trial 2 evaluated the effects of an IBV challenge on birds vaccinated against IBV. All birds were provided mash starter diets from 0 to 7 wk of age (18.1% CP; ME 2,996 kcal/kg). Two groups in each experiment had no Original XPC added to the feed (T1 and T2); T3 (XPC2) had 2.0 lb/ton of Original XPC included in the diet; T4 (XPC3) had Original XPC added at 3.0 lb/ton (recommended dose for starting layer pullets).
Trial 1: Primary Immune Response to IBV Challenge
At 21 d of age, all chicks, except the negative control (NC) group (Table 1), were challenged via the ocular and nasal routes with a wild Arkansas-type IBV virulent strain (105 EID50/bird; GenBank accession No. JN861120). Birds from each treatment were then evaluated for response according to the sample collection schedule outlined below on specified days post challenge (dpc).
Trial 1: Sample Collection
At 26 d (5 dpc), protection against IBV was evaluated from 20 birds/treatment by:
- Clinical signs;
- Viral load (lachrymal fluid [tears] and tracheal samples); and
- Trachea histopathology.
At 32 d (10 dpc), samples of the Harderian gland and spleen were collected from 10 birds/treatment and analyzed for:
- IBV responses; and
- Lymphocyte abundance.
Blood samples were collected from the remaining chickens (20 per treatment) on days 34, 41, and 48, and analyzed for antibodies to IBV.
Trial 2: Secondary Immune Response to IBV Challenge
Birds in this trial were vaccinated at 10 d of age via the ocular route with a commercially available IBV Massachusetts-type vaccine. All birds, except the NC treatment, were subsequently challenged at 25 d via the ocular and nasal routes with an Arkansas-type IBV virulent strain (105 EID50/bird), as described in Trial 1. Birds from each treatment were then evaluated for response according to the sample collection schedule outlined below at specified days post challenge.
Trial 2: Sample Collection
At 30 d (5 dpc), protection against IBV was evaluated from 20 birds/treatment by:
- Clinical signs;
- Viral load (lachrymal fluid [tears] and tracheal samples); and
- Trachea histopathology.
At 36 d (10 dpc), samples of the Harderian gland and spleen were collected from 10 birds/treatment and analyzed for:
- IBV responses; and
- Lymphocyte abundance.
Blood samples were collected from the remaining chickens (20 per treatment) on days 38, 45 and 52, and analyzed for antibodies to IBV.
Results: Trials 1 and 2
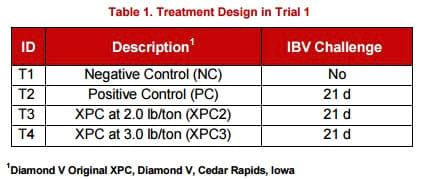
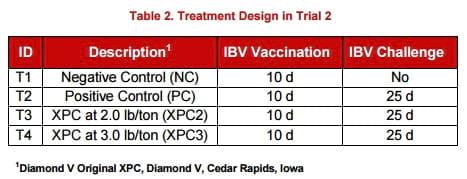
Clinical signs to IBV were statistically reduced at 5 dpc in both trials by the inclusion of Original XPC at the highest level (3.0 lb/ton), compared to the positive control (PC) group (Figure 1). No statistical differences were observed in mucosal thickness or deciliation of the trachea. Lymphocyte infiltration at 5 dpc was significantly lower (P < 0.05) in both Original XPC groups in Trial 1 compared to the PC treatment. No differences were observed in Trial 2 birds (Figure 2).
Viral load determined by qRT-PCR showed no differences in tears or the trachea at 5 dpc. This observation supports the premise that Original XPC does not kill viruses; rather, Original XPC helps support the immune system of birds, enabling them to protect themselves naturally.
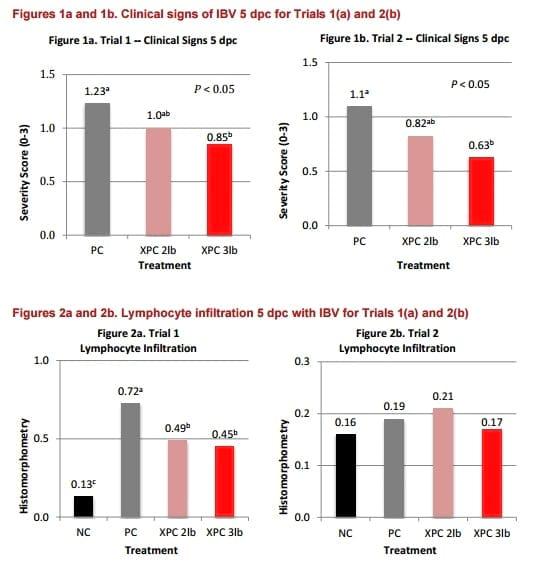
Previous research has shown increased secretory IgA levels in birds consuming Original XPC in the diet (Jensen et al., 2008 and Gao et al., 2008). At 10 dpc, splenic IgA lymphocytes were increased in XPC3 birds (3.0 lb XPC/ton, T4) of Trial 1 and Harderian gland IgA was elevated XPC3 birds (3.0 lb XPC/ton, T4) in Trial 2 compared to the PC treatment of each study (Figure 3). However, no rise in IgG levels was noted in either trial, which is consistent with previous work (Jensen et al., 2008 and Gao et al., 2008).
Cytotoxic cells as indicated by CD44+CD8+ markers were increased in the spleen of XPC3 birds (3.0 lb XPC/ton, T4) in Trial 1 at 10 dpc. No response was observed in the vaccinated groups of Trial 2.
Antibody titers to IBV showed higher levels in the XPC2 treatment (2.0 lb XPC/ton, T3) in Trial 2 compared to the PC group at 20 and 27 dpc. No differences were observed in Trial 1.
Conclusions
- Adding Original XPC (3.0 lb/ton) to experimental diets statistically reduced clinical signs of IBV in SPF chickens. The XPC2 dose (2.0 lb/ton) was intermediate. The recommended Original XPC Starter dose of 3 lb/ton was sufficient to reduce clinical signs of IBV.
- No differences were observed for mucosal thickness or deciliation in the trachea between treatments. Improvements in clinical signs were not a result of morphological changes in the trachea.
- No differences in viral load measured in tears or trachea indicates that Original XPC in the feed does not have a direct anti-viral effect. Rather Original XPC works indirectly through the bird’s immune system.
- IgA levels were elevated in XPC3 birds in both Trials. IgG levels were suppressed, and not significantly different from the PC birds. The increase of IgA with the suppression of IgG supports the concept that Original XPC balances the immune system of birds.
More recent research evaluating the effects of Original XPC on Newcastle Disease virus (NCDV) antibody titers indicates that birds fed Original XPC are likely to demonstrate an increase in antibody titers much earlier than control birds. In these trials, the earliest evaluation is at 13 dpc. This work shows that Original XPC-fed birds can have elevated titers to NCDV as early as 7 dpc.
References
- Al-Homidan, A. and M.O. Fahmy. 2007. The effect of dried yeast (Saccharomyces cerevisiae) supplementation on growth performance, carcass chemical analysis, immunity, ileum villi heights and bacterial counts of broiler chickens. Egypt Poult. Sci. 27:613-623.
- Fathi, M.M., S. Al-Mansour, I. Al-Homidan, A. Al-Khalaf. 2012. Effect of yeast culture supplementation on carcass yield and humoral immune response of broiler chicks. Vet. World 5:651-657.
- Gao, J., H.J. Zhang, S.H. Yu, S.G. Wu, I. Yoon, J. Quigley, Y.P. Gao, and G.H. Qi. 2008. Effects of yeast culture in broiler diets on performance and immune-modulatory functions. Poult. Sci. 87:1377-1384.
- Jensen, G.S., K.A. Redman, K.F. Benson, S.G. Carter, M.A. Mitzner, S. Reeves and L. Robinson. 2008. Antioxidant Bioavailability and Rapid Immune Modulating Effects after Consumption of a Single Acute Dose of a High-Metabolite Yeast Immunogen: Results of a Placebo-Controlled Double-Blinded Crossover Pilot Study. J. Med Food 14:1-9.
- Moyad, M.A., L.E. Robinson, J.M. Kittelsrud, S.G. Reeves, S.E. Weaver, A.I. Guzman, and M.E. Bubak. 2009. Immunogenic yeast based fermentation product reduces allergic rhinitis-induced nasal congestion: a randomized, double-blind, placebo controlled trial. Advances in Therapy 26:795-804.
- Moyad, M.A., L.E. Robinson, E.T. Zawanda, Jr., J.M. Kittelsrud, D.G. Chen, S.G. Reeves and S.E. Weaver. 2010. Immunogenic Yeast-Based Fermentate for Cold/Flu-like-Symptoms in Non-vaccinated Individuals. The J. of Altern. Complementary Med. 16(2):213-218.
Related topics
Authors:
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post







.jpg&w=3840&q=75)