Chickens: Feed supplementation to affect the rate and level of immune response
Published: August 10, 2015
By: Don McIntyre, Ph.D., PAS; John Carey, Ph.D.
The avian immune system consists of two components: Innate immunity and adaptive immunity. The innate portion of the immune system serves as the bird’s first line of defense against non-specific challenges. The adaptive side defends against more specific challenges that cross the intestinal wall or respiratory barrier and attack the bird systemically.
Published reports demonstrate feeding Diamond V Original XPCTM (XPC) has a beneficial impact on both the innate and adaptive immune system (Jensen et al., 2008; Moyad et al., 2009).
Vaccination is an important step in protecting animals from disease agents like viruses, parasites, and pathogenic bacteria. The success of any vaccine program relies on several factors, including timing and vaccine efficacy.
Newcastle disease virus (NDV) is globally recognized as a respiratory disease that can be devastating in its most virulent form, and may weaken birds’ immune defense in subclinical forms. The effects of feeding XPC on Newcastle vaccine titers have been reported in several broiler studies (Al-Homidan and Fahmy, 2007; Gao et al., 2008; Fathi et al., 2012). In each study, an improvement in vaccine titers was observed when birds were fed XPC, suggesting enhanced antibody production and potential increased protection against Newcastle disease.
However, the mechanism for increased antibody titers, a function of the adaptive immune system, has not been clearly defined. The current project was designed to improve our understanding of how XPC supplementation can influence the bird’s adaptive immune system.
Experimental Design
One-day-old Ross broiler chicks (n = 120) were divided into 2 feed treatments:
- T1 – control diet
- T2 – control diets plus XPC (2.5 lb/t)
Birds were grown at the Texas A&M University (TAMU) poultry research center. Standard broiler diets (Starter, Grower, and Finisher) and water were provided ad libitum to birds housed in floor pens from 0 to 42d of age.
Primary Immune Challenge
At 1d of age (prior to placement into pens), all chicks in both treatments were vaccinated with B1 Newcastle disease vaccine (NDV), followed by B1, Lasota NDV at 21d. All birds were vaccinated individually via nasal administration.
Sample Collection
Blood samples were collected from birds of each treatment (n = 15) at 14, 21, 28, 35 and 42d for antibody measurement. The same 15 birds per treatment were euthanized for collection of spleen, thymus, and bursa tissue. A standard procedure called flow cytometry was used to analyze blood and tissue cells to determine the immune competence of the birds’ adaptive immune response.
Flow Cytometry
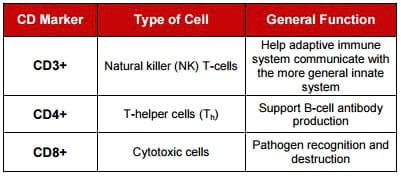
T cells belong to a group of white blood cells known as lymphocytes, which play a primary role in cell-mediated immunity. The "T", in T cell, stands for thymus since it is the principal organ for their development. Several different subsets of T cell (cytotoxic T cells, helper T cells, memory T cells, regulatory T cells, natural killer T cells) have been described, each with a distinct function. Similarly, “B” cell antibodies arise in the bursa and play a central role in humoral immune functions. T cells can be distinguished from other white blood cells, such as B cells and natural killer (NK) cells, by the presence of a special receptor on their cell surface called the Tcell receptor (TCR).
Through flow cytometry, cell differentiation can be determined by the affinity of lymphocytes to known markers like CD3+, CD4+ and CD8+. The CD4+/CD8- and CD4-/CD8+ T lymphocyte ratios were measured, and served as the immune-competence indicator of the adaptive immune response (T-helper cell vs. T-CTL cells).
Results
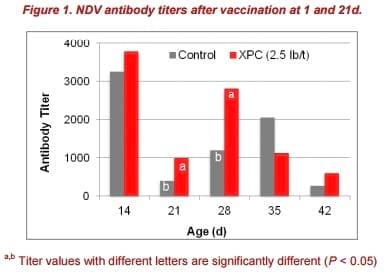
Antibody titers were significantly higher at 21 and 28d (P < 0.05) in XPC-fed birds, compared to the control group (Figure 1). Increased antibody titers following vaccination with NDV in birds fed XPC are consistent with previous reports (Al Homidan and Fahmy, 2007; Gao et al., 2008; Fathi et al., 2012).
Antibody titers were significantly higher at 21 and 28d (P < 0.05) in XPC-fed birds, compared to the control group (Figure 1). Increased antibody titers following vaccination with NDV in birds fed XPC are consistent with previous reports (Al Homidan and Fahmy, 2007; Gao et al., 2008; Fathi et al., 2012).
Furthermore, after the second vaccination at 21d, antibody titers in the XPC-fed group increased faster and reached a higher level than birds of the control group.
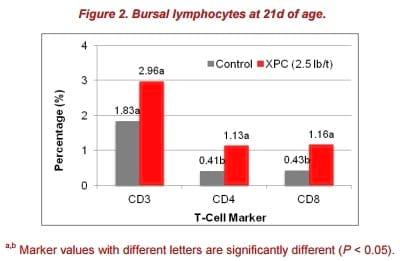
Also in this study, feeding XPC to broilers showed significant increases in bursa CD4+ and CD8+ at 21d of age (Figure 2). Higher B-cell proliferation rates seen in the XPC supplemented group indicated a more diverse B-cell repertoire. This effect also may have contributed to the robust humoral immune response observed in the antigen-specific antibody titers.
The interaction between T-helper cells and B cells in immune organs, such as the spleen, has a major impact on the development of antigen-specific B-cell responses during vaccination. Meanwhile, circulating T-helper cells have been demonstrated to enhance immunoglobulin production by B cells in mammals.
No statistical differences in peripheral blood cells between the control and XPC group were observed in this study. However, on 21d post-hatch, the XPC group had a numerically higher percentage of CD4+ T-cells (3.13% vs. 0.7%) in the blood compared with control. The proportion of positive staining T-cells dramatically increased at 14 and 28 d post-hatch and decreased on 21 and 42 d post-hatch. Thus, the 3.13% of CD4+ T-cells in the XPC group could partially contribute to the acceleration of B-cells, in turn leading to increased generation of antigen-specific antibodies. This effect could be one of the reasons that the antigen-specific antibody titer in the XPC group reached a peak on 28 days post-hatch, which is 7 days faster than the control group.
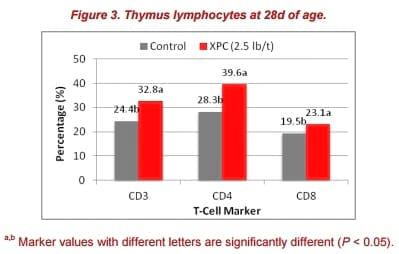
The XPC group also had a significantly higher percentage of CD3+, CD4+, and CD8+ T cells in the thymus at 28d (Figure 3). The thymus has been shown to be a sensitive immune organ following exposure to immune toxins and endogenous corticosteroids. An increase in lymphocyte numbers in the thymus is usually a response to antigenic stimulation. This observation was consistent with the immune response at 28d for antibody titers.
Conclusions
- Antibody titers were increased at 21 and 28d in birds fed Original XPC following NDV
- vaccination at 1 and 21d.
- Antibody titers peaked earlier in XPC-fed birds.
- Bursa lymphocytes associated with CD4+ and CD8+ were higher in XPC-fed birds at 21d.
- Immune markers (CD3+, CD4+ & CD8+) in the thymus were elevated for the XPC-fed group at 28d.
- Increased antibody titers and immune markers result in improved bird immune function and enhanced protection to viral challenge.
More to Come…
Additional data collected from this study will be reported in a future issue of PoultryAdvisor, describing: Immune organ development, histology, and gene expression profiles. Multiple genes were shown to be up-regulated in birds fed Original XPC to elicit these changes or in response to measured criteria.
References
- Al-Homidan, A. and M.O. Fahmy. 2007. The effect of dried yeast (Saccharomyces cerevisiae) supplementation on growth performance, carcass chemical analysis, immunity, ileum villi heights and bacterial counts of broiler chickens. Egypt Poult. Sci. 27:613-623
- Fathi, M.M., S. Al-Mansour, I. Al-Homidan, A. Al-Khalaf. 2012. Effect of yeast culture supplementation on carcass yield and humoral immune response of broiler chicks. Vet. World 5:651-657
- Gao, J., H. J. Zhang, S. H. Yu, S. G. Wu, I. Yoon, J. Quigley, Y. P. Gao, and G. H. Qi. 2008. Effects of yeast culture in broiler diets on performance and immune-modulatory functions. Poult. Sci. 87:1377-1384
- Jensen, G.S., K.A. Redman, K.F. Benson, S.G. Carter, M.A. Mitzner, S. Reeves and L. Robinson. 2008. Antioxidant bioavailability and rapid Immune modulating effects after consumption of a single acute dose of a high-metabolite yeast immunogen: Results of a placebo-controlled double-blinded crossover pilot study. J. Med Food 14:1-9
- Moyad, M.A., L.E. Robinson, J.M. Kittelsrud, S.G. Reeves, S.E. Weaver, A.I. Guzman, and M.E. Bubak. 2009.
- Immunogenic yeast based fermentation product reduces allergic rhinitis-induced nasal congestion: a randomized, double-blind, placebo controlled trial. Advances in Therapy 26:795-804
Related topics:
Authors:
Diamond V
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.








.jpg&w=3840&q=75)