Clostridium difficile in Piglets
Clostridium difficile in a farrowing pen
Published: January 5, 2012
By: L.J.A Lipman, Hopman N.E.M., E.C. Keessen (Utrecht University)
Abstract
Clostridium difficile is an important cause of enteric disease in humans. In pigs Clostridium difficile can cause neonatal enteritis and can be isolated from faeces from diseased and healthy animals. According to recent research, isolates from humans and animals show genetic and phenotypic overlap. In The Netherlands, strains isolated from diseased piglets were indistinguishable from strains isolated from Dutch patients. These strains belonged to ribotype 078. Because pigs can either be clinical hosts and/or may be a possible reservoir more understanding of the epidemiology of Clostridium difficile among pigs is needed. The objectives of this study were to specify whether, how and when newborn piglets get infected by Clostridium difficile for the first time. With this intention, six sows, their farrowing crates and litters (71 piglets) at one farm were sampled around the day of birth of the piglets. Within 48 hours after birth, all sampled 71 piglets at the farm became positive for Clostridium difficile ribotype 078. Moreover, all sows became positive within 113 hours after birth of the piglets and the farrowing crates were intermittently positive during the sampling period. This research shows that the sow, the farrowing crate, the air and the teats of the sow are possible transmission routes of Clostridium difficile ribotype 078.This information might help to advise farmers on taking measures against Clostridium difficile infections in neonatal piglets.
Clostridium difficile is an important cause of enteric disease in humans. In pigs Clostridium difficile can cause neonatal enteritis and can be isolated from faeces from diseased and healthy animals. According to recent research, isolates from humans and animals show genetic and phenotypic overlap. In The Netherlands, strains isolated from diseased piglets were indistinguishable from strains isolated from Dutch patients. These strains belonged to ribotype 078. Because pigs can either be clinical hosts and/or may be a possible reservoir more understanding of the epidemiology of Clostridium difficile among pigs is needed. The objectives of this study were to specify whether, how and when newborn piglets get infected by Clostridium difficile for the first time. With this intention, six sows, their farrowing crates and litters (71 piglets) at one farm were sampled around the day of birth of the piglets. Within 48 hours after birth, all sampled 71 piglets at the farm became positive for Clostridium difficile ribotype 078. Moreover, all sows became positive within 113 hours after birth of the piglets and the farrowing crates were intermittently positive during the sampling period. This research shows that the sow, the farrowing crate, the air and the teats of the sow are possible transmission routes of Clostridium difficile ribotype 078.This information might help to advise farmers on taking measures against Clostridium difficile infections in neonatal piglets.
Introduction
Clostridium difficile (CD) is an anaerobic Gram-positive, spore-forming bacterium. It is widely distributed and can be found in soil, water, intestinal tracts of animals, and even on meat CD has been found in a wide variety of animal species e.g. pigs, calves, dogs, horses, ostriches, elephants, ratites, cats and mice.
Isolation by culturing and toxin detection are the main methods used in the laboratory for diagnosis of CD associated disease (CDAD). For typing of CD various techniques are used, as PCR ribotyping, pulsed-field gel electrophoresis and toxinotyping (Weese, 2010). Using PCR-ribotyping, CD can be divided in more than 300 different ribotypes.
In piglets, an infection with CD can cause neonatal enteritis. Pigs, 1-7 days old, are affected and may show diarrhoea, although, some pigs are obstipated. (Songer et al., 2006) The CD ribotype mostly found in neonatal piglets in The Netherlands is ribotype 078 (Keessen et al., 2010).
Debast et al. (2009) isolated CD ribotype 078 strains from diseased piglets from two Dutch pig-breeding farms with problems of neonatal diarrhoea and from Dutch human patients. The ribotype 078 strains were indistinguishable. Therefore a common origin of human and animal strains could be considered.
Because pigs can be either clinical hosts of CD and/or possible reservoirs for humans, more understanding of the epidemiology of CD among pigs is needed. The objective of this study was to determine how soon after birth, CD ribotype 078 could be isolated from newborn piglets. In addition, the newborn´´´´s environment, e.g. sows and farrowing crates, were sampled for CD to determine by which routes piglets get infected.
Materials and methods
Sampling
Sampling
The research was conducted at a Dutch pig breeding farm with 200 sows, where Clostridium difficile ribotype 078 was known to be present. Sows, the environment and piglets (normally born or after caesarean section) were sampled. Sampling before and after normal parturition: at the moment the sows (6) entered their farrowing crate, approximately one week before parturition, a stool sample of the sow and a sample of the farrowing crate were collected. Within 15 hours after birth of the piglets, the sow, its environment and the neonatal piglets were sampled. A rectal stool sample was taken of the sow, the environment was sampled using electrostatic cloths and piglets were sampled using rectal swabs. All 72 piglets from these 6 litters got an ear tag and were monitored for illness, as diarrhoea, during the sampling period.
Subsequently, the newborn piglets, the sow and the environment were sampled once a day until Clostridium difficile was detected. The teats of the sow and the ambient air of the farrowing compartment were infrequently sampled. Another six sows and their environment were sampled before delivery; their piglets were not monitored, because first samples of the newborn piglets could not be taken within 15 hours post partum. Sampling is described in Hopman et al, 2011.
Culturing
Culturing
All rectal and environment samples were cultured for CD using an enrichment broth as described by Rodriguez-Palacios et al. (2007). After enrichment in Clostridium difficile moxalactam norfloxacin (CDMN) broth (broth produced by Mediaproducts, The Netherlands), the culture broth was homogenized and two ml was transferred into a sterile tube. The broth was mixed with two ml 96% ethanol and left at room temperature for >60 minutes (alcohol shock to for bacterial spores). After centrifugation (4000 x g for 10 min), the supernatant was discarded and the sediment was plated onto commercially-prepared Clostridium difficile agar (Clostridium difficile agar (CLO agar), Biomérieux). These culture plates were incubated anaerobically, using gaspaks (GasPak EZ Anaerobe Container System Sachets, BD) and anaerobic jars, at 37 oC for at least 48 hours.
Colonies characteristic for CD were identified by morphological criteria, the characteristic horse-manure odour and Gram-staining. Confirmation of identification of CD was done at the University Medical Center in Leiden. Genetic identification of CD was done by an in-house PCR for the presence of the gene encoding glutamate dehydrogenase (gluD) specific for CD. All strains were further investigated by PCR-ribotyping.
Results
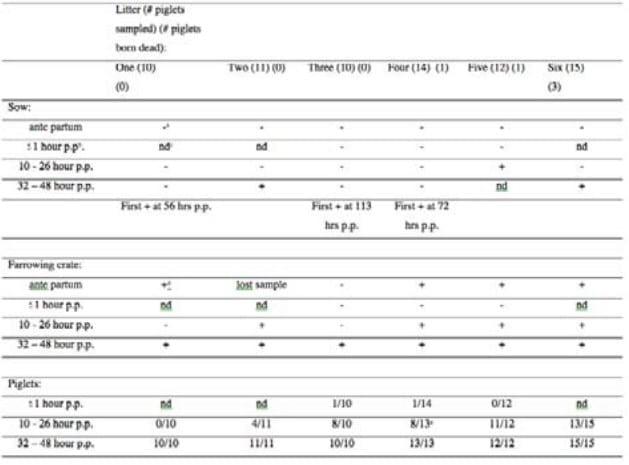
Presence of Clostridium difficile 078 in sows ante en post partum, piglets and the farrowing crate ante partum are described in table 1.
Table 1
Presence of Clostridium difficile ribotype 078 in sows, her individual farrowing crate and her piglet
Results
Presence of Clostridium difficile 078 in sows ante en post partum, piglets and the farrowing crate ante partum are described in table 1.
Table 1
Presence of Clostridium difficile ribotype 078 in sows, her individual farrowing crate and her piglet
a (-) CD 078 not found after culturing, b (p.p.) post partum, c (nd) not done, d (+) CD 078 found after culturing, e one piglet died On teats of two sows, Clostridium difficile 078 was found ante partum. All samples of the teats taken post partum, were positive for CD 078. Ante partum the air had been sampled in one farrowing pen and was found to be positive for CD 078. All air samples of the farrowing pens, taken post partum, were positive for CD 078 at the moment of sampling.
Discussion and conclusion
This study demonstrated that all sampled newborn piglets, irrespective of the presence of diarrhoea, got infected with CD ribotype 078 within two days after birth. Within this herd, just one ribotype, CD 078, was isolated, not only from neonatal piglets, but also from sows, ambient airsamples, and from the environment of the piglets. CD seems to be able to spread easily between sows, piglets and the environment. In the present study, only one ribotype was found. It is possible that pigs are infected with more ribotypes and that more ribotypes are present at the farm but that the isolation techniques are more sensitive for CD ribotype 078 and other ribotypes are overlooked. Our findings suggest that all piglets become infected with one ribotype. Little is known about the spread, contamination and infection of CD through aerosols in piglets. At the selected farm, the farrowing pens were always cleaned, after weaning of the piglets, using alkaline foam cleaner.
Occasionally, Halamid® was used. CD 078 was found on the floor, in the air and under boots (data not shown), so it might be concluded that the cleaning and disinfection protocol used at this farm, is inadequate to kill CD or the protocol is not executed properly. To control CD 078 at farms, effective hygiene and disinfection procedures should be established. Environmental disinfection should be performed using sporocidal agents, which ideally contain chlorine.
References
Debast, S.B et al, 2009. Clostridium difficile PCR ribotype 078 toxinotype V found in diarrhoeal pigs identical to isolates from affected humans. Environ. Microbiol. 11, 505-511.
Hopman, N. et al, 2011. Acquisition of Clostridium difficile by piglets. Vet Microbiol. 149, 186-92.
Keessen, E. et al,2010. Aanwezigheid van Clostridium difficile in biggen verdacht van CDI op elf varkensbedrijven in Nederland. Tijdschrift voor Diergeneeskunde. 135, 134-137.
Rodriguez-Palacios, A., et al, 2006. Clostridium difficile PCR Ribotypes in Calves, Canada. Emerg. Infect. Dis. 12, 1730-1736.
Songer,et al, 2006. Clostridium difficile: An important pathogen of food animals. Anaerobe. 12, 1-4.
Weese, J.S., et al, 2010. Longitudinal investigation of Clostridium difficile shedding in piglets. Anaerobe. 16, 501-504
Debast, S.B et al, 2009. Clostridium difficile PCR ribotype 078 toxinotype V found in diarrhoeal pigs identical to isolates from affected humans. Environ. Microbiol. 11, 505-511.
Hopman, N. et al, 2011. Acquisition of Clostridium difficile by piglets. Vet Microbiol. 149, 186-92.
Keessen, E. et al,2010. Aanwezigheid van Clostridium difficile in biggen verdacht van CDI op elf varkensbedrijven in Nederland. Tijdschrift voor Diergeneeskunde. 135, 134-137.
Rodriguez-Palacios, A., et al, 2006. Clostridium difficile PCR Ribotypes in Calves, Canada. Emerg. Infect. Dis. 12, 1730-1736.
Songer,et al, 2006. Clostridium difficile: An important pathogen of food animals. Anaerobe. 12, 1-4.
Weese, J.S., et al, 2010. Longitudinal investigation of Clostridium difficile shedding in piglets. Anaerobe. 16, 501-504
This presentation was given at SafePork International Conference, June 19-22, 2011 in Maastricht, The Netherlands. Engormix.com thanks the authors and the organizing committee for this contribution.
Related topics
Authors:
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post2 de febrero de 2012
It is combined microbial composition containing the association of microorganisms from natural micro biota. Trials of this compound were held in 3 pig farms. We are ready to collaboration. best regards A. Buzun (epibuz2@yahoo.co.in)
Utrecht University
2 de febrero de 2012
I am interested in this agent. Based on phages or what? Has it been tested already in farm conditions? Maybe we can test it in our university farm. kind regards Len Lipman (l.j.a.lipman@uu.nl)
1 de febrero de 2012
Chemical disinfection is inadequate to kill CD & CP. We have hopeful results with some biological agent for inhibition of CP in piggery.
A.Buzun







.jpg&w=3840&q=75)