Artificial Insemination in Pigs
Published: December 26, 2013
By: Maes Dominiek, López Rodríguez Alfonso, Rijsselaere Tom,
Vyt Philip and Van Soom Ann (Ghent University, Faculty of Veterinary Medicine)
Belgium
1. Introduction
Artificial insemination (AI) of swine is widely practiced in countries with intensive pig production. In Western Europe, more than 90% of the sows have been bred by AI for more than two decades (Gerrits et al., 2005; Vyt, 2007). When compared with natural mating, AI is a very useful tool to introduce superior genes into sow herds, with a minimal risk of disease (Maes et al., 2008). The outcome of AI largely depends on the semen quality and the insemination procedure. In practice, fresh diluted semen for intracervical insemination is mostly used in pigs. Semen is obtained from boars on farms or from specialised AI-centres. The latter offer a diversity of breeds and genetic lines and distribute ready-to-use semen doses of constant quality to different sow herds.
Three important aspects should be considered. Firstly, only semen from healthy boars should be used, as diseased boars may ejaculate semen that is contaminated with pathogens. The semen from commercial AI-centres is shipped to a large number of sow farms. Contaminated semen could therefore lead to a rapid transmission of pathogens and to disease outbreaks in many different sow herds. Strict regulations and guidelines to prevent disease spreading are therefore implemented on porcine AI-centres. The second important aspect is the fertilizing capacity of the produced semen doses. The fertilizing potential of a semen dose is inherently linked to the quality of the spermatozoa itself (Tsakmakidis et al., 2010). Examination of the ejaculates is therefore necessary. A third important aspect of AIcentres is the semen processing procedure (Waberski et al., 2008). This is not only important to guarantee a low microbial presence but even more to obtain high quality sperm, namely viable spermatozoa in ready-to-use semen doses that can be used for several days. The dilution procedure and semen handling, the properties of the extender and the microenvironment for the sperm cells influence the survival and longevity of the spermatozoa.
The present chapter will review and critically discuss the different steps during the entire AI procedure in pigs, starting from the semen collection, dilution and processing, methods and technologies used to assess the semen quality, the storage conditions and the characteristics of the semen extenders that are required to maintain semen quality. A last part will focus on the different AI strategies.
2. Collection of semen, dilution and processing
Although automated semen collection systems have been developed (Barrabes Aneas et al., 2008), semen is mostly collected by the gloved hand technique from a boar trained to mount a dummy sow. Dummy sows should be solid in construction without sharp edges, and located in a quiet designated semen collection room with a non-slippery floor. A prewarmed (38°C) collection container is used. The top of the container is covered with cheesecloth to filter out gel portion of the semen. The end of the penis is grabbed firmly with a gloved hand and the collection process is initiated with firm pressure to the spiral end of penis with the hand so that the penis cannot rotate. This process imitates the pressure applied by the corkscrew shape of the sow’s vagina. Polyvinyl gloves can be used, not latex gloves as these are toxic for the semen (Ko et al., 1989). The first part of the ejaculate (presperm) should be discarded. It is clear, watery fluid and does not contain sperm (~25 ml), but it may have a high bacterial count. The sperm-rich fraction should be collected (40-100 ml). It is very chalky in appearance and contains 80-90% of all sperm cells in the ejaculate. Once the sperm-rich fraction is complete, the remainder of the ejaculate is again more clear, watery fluid, and should not be collected (70-300 ml). After collection, the filter with gel should be discarded, and the collection container should be placed in warm water. The semen should be extended within 15 min. after collection. The ejaculation lasts up to 5 to 8 min, but may continue up to 15 min. About 100 to 300 ml of semen is collected. Semen collection from boars in AI-centres is performed approximately 2 times per week (Vyt et al., 2007).
The extension process should be done in a warm room with clean and sterile equipment. The extender is added to the semen, and cold shock should be avoided by diminishing the temperature gradually. A normal ejaculate usually contains enough sperm to inseminate 15 to 25 sows using conventional AI. Each dose should contain 2-3 billion spermatozoa in 80- 100 ml.
3. Semen quality assessment
3.1 Assessment of the concentration of spermatozoa in the ejaculate
The number of spermatozoa in a semen dose is important for the fertilization process. On the other hand, AI-centres tend to dilute the ejaculates as much as possible to maximize semen dose production. Variation in the number of spermatozoa in an ejaculate has been described between different pig breeds e.g. Landrace, Duroc and Yorkshire (Kommisrud et al., 2002), which is a first factor influencing semen dose production. Not only differences in sperm number but also in sperm volume, ranging from 100 to 300 ml (Kondracki, 2003), influence sperm concentration. Individual variation within a breed is also very important (Johnson et al., 2000). Xu et al. (1998) demonstrated a difference in litter size of 0.09 to 1.88 piglets when inseminating sows either with 2*109 or 3*109 spermatozoa. Differences in litter size between both semen doses were largely dependent on individual variations between boars. Another study (Alm et al., 2006) using 2*109 spermatozoa per dose mentioned not only a smaller litter size but also a lower farrowing rate at lower semen dose. In addition, several studies (Alm et al., 2006; Xu al., 1998) described lower fertility results when lower semen doses were used in boars with suboptimal semen quality. A general guideline for the number of good quality spermatozoa (Table 1) in a semen dose was set at 3*109 spermatozoa per dose. According to the morphological or motility characteristics, the number of spermatozoa should be adapted (Martin-Rillo et al., 1996). By multiplying the total volume of the gel-free ejaculate (ml) times the sperm concentration per ml, the total sperm number is calculated. The volume is routinely measured by weighing the ejaculate considering 1 gram equal to 1 ml and the obtained total sperm numbers is a good indicator to evaluate spermatogenesis (Amann, 2009).The data above clearly indicate the importance of an accurate determination of the concentration of spermatozoa in the ejaculate.
3.1.1 Inspection of the raw ejaculate
Visual evaluation of the opacity of the ejaculate gives a rough idea on the sperm concentration. However, this method is crude and very subjective and therefore not suitable for AI-centres with large semen production.
3.1.2 Counting chambers
Different glass chambers are described to count cells in a known volume. Haemocytometers, such as the Neubauer, Thoma and Bürker chamber are reusable glass chambers with fixed volume used for counting immobilized spermatozoa in a grit. Other reusable glass chambers as the Mackler chamber are used for assessing concentration as well as motility (Tomlinson et al., 2001). Disposable chambers (MicrocellR, LejaR) are commonly used in Computer Assisted Semen Analysis (CASA) since their small depth limits movement in the third dimension (Z-axis) when the sperm path is analysed (Verstegen et al., 2002). Haemocytometers are considered as the standard method for determining sperm concentration and have a lower coefficient of variation than disposable chambers (Christensen et al., 2005; Tomlinson et al., 2001). The concentration determined by the haemotocytometer however, was generally higher than the concentration determined using other chambers, especially with increasing concentration. Makler chambers were described as having higher standard deviations and more inconsistent results compared with the haemocytometer (Christensen et al., 2005; Tomlinson et al., 2001). Disposable chambers on the other hand, although they are also used for counting live cells, were reported to be more consistent and accurate (Mahmoud et al., 1997). The accuracy of different counting chambers is also dependent on the manner in which the chamber is filled. Thin, capillary-filled, disposable chambers are generally found to underestimate sperm concentration due to the Segre-Silberberg effect (Kuster, 2005). However, the variations between chambers when analysing sperm concentration seems to be technician and laboratory dependent (Christensen et al., 2005).
3.1.3 Photometry
Photometers (single wavelength) or spectrophotometers (multiple wavelengths) measure the optical density, i.e. the relative absorption and scattering of a light beam that is sent through a semen sample. The absorption and scattering is proportional to the sperm concentration. Next to the concentration of spermatozoa, the absorbance is also influenced by gel particles in the seminal plasma or the extender, by the quality of the sample cuvette, and the dilution of the sample (Knox, 2004). Photometry is commonly used in practice because it is fast and easy to perform (Woelders, 1991). Accurate dilution and a correct calibration curve are imperative to obtain proper results.
3.1.4 Flow cytometry
Several studies use fluorescent dyes that stain intact or damaged spermatozoa differently, and measure the distribution of dyes in the sperm cell population by a flow cytometer (Christensen et al., 2004; Ericsson et al., 1993). In that way, viability as determined by the percentage of intact cells as well as the concentration of spermatozoa in an ejaculate can be determined by using fluorescent microspheres. Since this technology can discriminate interference from gel particles, it has a low coefficient of variation (3.3%) (Christensen et al., 2004). However, the high costs and the dependence on qualified personnel make flow cytometry not the most suitable method for use in practice.
3.1.5 Other methods
Coulter counters, determining the number of particles within a known volume, can be used to assess spermatozoa concentration but discrimination of other particles with comparable size within the sample is difficult, resulting in a lower accuracy (Woelders, 1991). Other systems, e.g. computer assisted semen analysis (CASA) use image analysis to determine sperm concentration within counting chambers (Prathalingam et al., 2006; Verstegen et al., 2002). The accuracy of these systems depends not only on the optical properties and the software settings, but also on the kind of counting chamber that is used (Kuster, 2005). Nucleocounters are also used for determining sperm concentration, and they provide similar counts as those obtained with photometers (Camus et al., 2011; Hànsen and Hedeboe, 2003). In these devices, DNA is fluorescently labelled and counted by image analysis resulting in an accurate determination of sperm concentration.
The concern to obtain a correct estimate of sperm concentration led to a discussion on the accuracy of the different systems. Maes et al. (2010) did not find major differences between two types of colorimeters, the Bürker counting chamber, and the Hamilton Thorne Analyzer (Ceros 12.1) using two Leja chambers. Every system has its advantages and limitations. They concluded that in commercial porcine AI-centres, economic considerations such as purchase prices, labour, and high sample throughput are also important in the choice for one method or the other.
3.2 Morphology and viability assessment
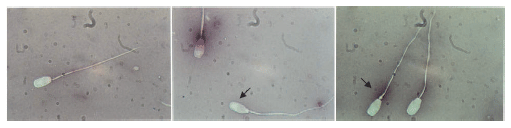
The microscopic appearance of spermatozoa can give information on morphological abnormalities, cell membrane integrity and the acrosome. These are three important parameters that contribute to the fertilizing capacity of the sperm cells. Morphological abnormalities give an indication of aberrations in the spermatogenesis. Some malformations compromise the function of the cells and cannot be compensated for, therefore leading to culling of the boar. Abnormal shape of the head which carries the genetic material or abnormalities of the mitochondrial sheet which is important for the function of the flagella, are therefore called primary abnormalities. Remainders of cytoplasm, proximal or distal droplets, and small tail abnormalities are called secondary abnormalities and can be compensated for by the semen dose (Donadeu, 2004). Additionally, morphological anomalies (e.g. coiled tails) acquired by inappropriate handling of semen are called tertiary abnormalities.
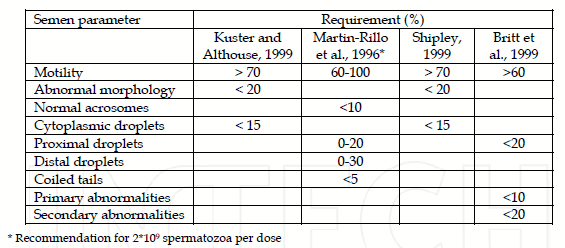
Morphology can be assessed by staining techniques that do not require highly qualified personnel (Shipley, 1999). Normal morphology is correlated with fertility (Alm et al., 2006; Xu. et al., 1998), and should therefore be performed routinely in porcine AI-centres. Criteria for the maximum percentage of primary and secondary abnormalities in commercial porcine AIcentres were determined as 10% and 20%, respectively (Shipley, 1999). The percentage of spermatozoa with normal morphology should be at least 70% (Shipley, 1999). An overview of the criteria for use of porcine semen in artificial insemination is shown in Table 1.
Table 1. Overview of the cut-off values for porcine semen quality in artificial insemination
Although several stains can be used, staining spermatozoa of farm animals for morphological examination is usually combined with membrane integrity assessment using a dye that is excluded by live cells, such as eosin (Figure 1). Therefore, besides being helpful for assessing sperm morphology, the eosin-nigrosin stain can be used to discriminate between live and damaged cells. This staining technique is widely used and is considered a simple and reliable technique that is easy to applyand its outcome correlates with fertility (Tsakmakidis et al., 2010).
Next to visual morphology assessment, automated CASA-systems were developed to obtain more objective information (Rijsselaere et al., 2005; Verstegen et al., 2002). Automated Sperm Morphology Analysis systems (ASMA) are able to locate the head of the spermatozoa and compare its morphology to internal standards. A disadvantage is the lengthy analysis which is required for each sample, and which is partly dependent on the contrast of the cells from the background necessary for the system to recognise the cell. The prolonged analysis time undoes the advantage of an objective measurement and renders the method not suitable for use in commercial AI-centres at the moment.
Several fluorescent dyes can also be used to assess cell membrane integrity and can be combined with flow cytometric analysis (Althouse and Hopkins, 1995; Woelders, 1991). The need for qualified personnel and a fluorescence microscope excludes the use of the latter stains from commercial AI-centres, although attempts have been made to incorporate this technology in the most recent generation of CASA-instruments. Next to staining methods, membrane integrity can also be evaluated by testing the osmotic resistance of the cells (Donadeu, 2004). The osmotic resistance of the porcine sperm cells was correlated with fertility results (Pérez-Llano et al., 2001). More advanced methods measure cell volume by detecting voltage changes when cells pass a capillary pore in a CASY 1 cell counter (Petrunkina al., 2004). Osmotic reactivity is a sensitive detection method of changes in plasma membrane, either in damaged cells or in capacitating cells.
Since the acrosome is important for the penetration of the oocyte, its integrity is considered vital for an optimal fertilising capacity. The acrosome integrity can be evaluated on the basis of its microscopic appearance, either by phase contrast evaluation of glutaraldehyde fixed spermatozoa or by various staining methods (de Andrade et al., 2007; Woelders, 1991).
Fig. 1. Sperm morphology: spermatozoa with normal morphology, abnormal (narrow) head (primary defect) and proximal droplet (secondary defect) (arrows)
3.3 Motility assessment
Motility of spermatozoa has always been considered a primary requirement to fertilize eggs. Although the spermatozoa are brought to the fertilization site mainly by uterine contractions (Langendijk et al., 2002), sperm motility is required for penetration of the zona pellucida. Motility is known to be an important characteristic in predicting the fertilizing potential of an ejaculate (Gadea, 2005). Therefore, several methods have been used for motility assessment.
3.3.1 Visual motility estimation
The simplest way to evaluate sperm motility is by estimating the number of motile spermatozoa under a light microscope or using phase contrast microscopy. This method is subjective since it depends on the interpretation by an individual (Vyt et al., 2004b). It is however a cheap method and facilitates a high sample throughput which makes it popular in commercial AI-centres.
3.3.2 Computer assisted semen analysis (CASA)
Using digital image analysis, sperm cell tracks are analysed in different components (Rijsselaere et al., 2003; Verstegen al., 2002; Vyt et al., 2004b). CASA has major advantages: the method is objective, independent of the interpretation of the technician and gives detailed information on the sperm movement. This way, different motility patterns can be observed, e.g. progressive movement versus hyperactivity and even different subpopulations of spermatozoa within an ejaculate can be demonstrated (Peña et al., 2005; Rijsselaere et al., 2005; Verstegen et al., 2002). The detailed information given by the CASAsystems renders them also very susceptible to external influences on sperm movement, such as operator variability, semen handling and system settings are causes of inter-laboratory differences (Rijsselaere et al., 2003; Verstegen al., 2002). At the moment, CASA instruments have been validated for many animal species (Holt et al., 1994, 1996; Rijsselaere et al., 2003; Wilson-Leedy and Ingermann, 2007) which makes the method available for use in veterinary practice or commercial AI-centres. The high cost of the equipment compared to the alternative visual motility determination, is a restraint to the use of CASA in practice.
3.3.3 Sperm Quality analyzer (SQA)
The SQA systems convert variations in optical density into electrical signals to determine sperm concentration and motility. These electronic signals are analyzed by the SQA software algorithms and translated into sperm quality parameters. The effectiveness of different SQA systems for sperm analysis has been studied both in humans and animals, and different algorithms are needed for each species. A previous version of the SQA namely the SQA-IIC was consistent and suitable for the estimation of boar semen quality. There was a good correlation between the sperm motility index (SMI) obtained by SQA-IIC and several CASA parameters, especially with the percentage of motile sperm and with straight line velocity (VSL). However, the SQA-IIC is based on an old technology meant for human sperm analysis and the SMI values are based on overall information of the quality of the sperm, and do not discriminate between concentration, morphology and motility parameters. Recently, the SQA-Vp was introduced as an SQA device specifically designed for boars in which the sperm movement can be visualized on a screen and motility is given as percentage of motile sperm (López et al., 2011).
In pigs, a motility score of 60% motile cells, independent of the method of assessment, is required to be considered as a fertile ejaculate (Donadeu, 2004). Above 60% motile spermatozoa, no differences in farrowing rate and litter size were recorded (Donadeu, 2004). Apart from morphology, several attempts were made to correlate motility with fertility outcome. When using adequate numbers of spermatozoa per insemination dose (3*109), correlation with fertility outcome was hard to establish (Gadea al., 2004). At lower semen dose, motility was well-correlated with fertility parameters. In most studies involving pigs, the predictive effect of motility was evaluated using visual motility assessment. To increase the discriminating power of the motility estimation, objective motility assessment by CASAmeasurements (Holt et al., 1997; Vyt et al., 2008) or motility of spermatozoa subjected to a percoll gradient (Popwell and Flowers, 2004) were used. These studies found motility to be positively correlated with fertility, especially with litter size.
3.3.4 Other sperm examination techniques
Sperm examination techniques requiring specialized knowledge and expensive equipment are not frequently used in commercial AI-centres. They are however used for research on porcine sperm. DNA fragmentation tests can be used to identify subfertile boars, but the study results are contradictory (Waberski et al., 2011; Boe Hansen et al., 2008). Some metabolic responses of sperm like resistance to oxidative stress (López et al., 2010) and in vitro fertilisation assays are also used for research purposes. The practical relevance of these techniques is limited due to the fact that most of the research on porcine semen is based on the semen from good performing boars (López et al., 2010). Subfertile or nonfertile boars are rapidly culled because of economic considerations, and therefore, there is a lack of information regarding sperm quality of infertile boars.
4. Storage of liquid semen
Frozen storage of boar semen still yields inferior fertility due to the loss of membrane integrity during freezing and thawing. Consequently, freshly diluted semen (liquid semen) is widely used for AI on the day of collection or in the following days. For storage of liquid boar semen, two factors are very important: the temperature of collection and storage, and the composition of the storage medium (Johnson et al., 2000).
4.1 Temperature of collection, transport and storage
A different composition of the phospholipids in the membrane of boar spermatozoa compared to bull spermatozoa, a low cholesterol/phospholipid ratio and an asymmetrical distribution of cholesterol within the membrane render boar spermatozoa very susceptible to cold temperatures resulting in increased permeability and loss of controlled membrane processes (De Leeuw et al., 1990). Hence, rapid cooling of ejaculates to 15°C or cooling below 15°C results in loss of viability or cold shock (Johnson et al., 2000). To avoid this cold shock, prediluted ejaculates are better left at temperatures above 15°C for several hours to induce cold resistance. In practice, semen is collected in isolated cans to avoid contact with colder surfaces and subsequent dilution is done in a manner in which temperature is diminished gradually. Two different protocols are normally used for this purpose: 1) One step dilution with either preheated diluter (~33°C) or diluter at room temperature or 2) a two steps dilution with first a 1:1 dilution with preheated diluter (~33°C), followed by a second dilution in either a preheated diluter or a diluter kept at room temperature (Waberski, 2009). After the final dilution, filling of commercial doses is done and the semen is allowed to cool down gradually to 17°C. When semen doses are to be transported, special precautions are taken to avoid temperature fluctuations (Green and Watson, 2002). Further storage of diluted semen is done at 17°C, at which temperature semen metabolism is reduced (Althouse et al., 1998), a condition necessary to extend storage time.
4.2 Storage medium
The storage media for liquid boar semen aim to prolong sperm survival, to provide energy to the cells, to buffer the pH of the suspension and to avoid the growth of bacteria (Vyt et al., 2004a). Therefore, porcine semen extenders contain ions to maintain the osmotic pressure of the medium, glucose as energy source, buffering systems to stabilize the pH of the extender and EDTA and antibiotics to prevent bacterial overgrowth (Johnson et al., 2000). The presence of glucose as the only energy source and the low oxygen content in the recipient in which diluted semen is stored stimulates the glycolytic metabolism. Consequently, the intracellular pH of spermatozoa is lowered which reduces their motility and enables them to survive several days at ambient temperature (Henning et al., 2009). Glucose also contributes largely to the osmotic equilibrium. The ions in the media for liquid boar semen are merely sodium bicarbonate and sodium citrate and are simultaneously used as buffer. In BTS extender, also KCl is added to prevent the potassium loss from inside the cells, and subsequent loss of motility due to Na-K pump inefficacy. Porcine spermatozoa are rather tolerant to minor changes in osmolality of the extender (Johnson et al., 2000). Iso-osmotic and slightly hyper-osmotic media are preferred for optimal preservation of fertilizing capacity (Weitze, 1990). Incubation in media below 250 mOsm and above 300 mOsm rendered irreversible damage to the membranes and subsequent loss of motility.
EDTA is added for its chelating properties. When Ca-ions are captured, the initiation of capacitation is inhibited (Watson, 1995). As a consequence, the fertilizing capacity of the spermatozoa is preserved. Depending on the composition of the extender, semen can be stored for 2 to 3 days in short-term extenders and up to five days or longer in long-term extenders (Johnson et al., 2000). Long-term extenders differ from short-term extenders mainly by the use of complex buffering systems (HEPES, Tris), mostly in addition to the bicarbonate buffering system, and by the presence of Bovine Serum Albumin (BSA). The latter has a positive influence on sperm survival due to the absorption of metabolic bacterial products from the extender. Cysteine is used as a membrane stabilizer (Johnson et al., 2000) inhibiting capacitation.
To prevent bacterial proliferation during storage, antibiotics are added to the extender. Bacteria originate mostly from the prepuce, thus depending on the semen collection technique, from semen manipulation or from the water used in the extender preparation (Althouse and Lu, 2005). Depending on the species, bacteria have deleterious effects on semen quality, namely depressed motility, cell death and agglutination (Althouse al., 2000), either by direct effect on the spermatozoa or by acidifying the environment. European legislation prescribes an antibiotic combination equivalent to 500 IU/ml penicillin, 500 IU/ml streptomycin, 150 mg/ml lincomycin and 300 mg/ml spectinomycin, for having a broad antibacterial spectrum and activity towards leptospira. In practice most commercial extenders use aminoglycosides, especially gentamycin (Althouse and Lu, 2005; Vyt et al., 2007). However, bacterial contamination should be first minimized by good hygiene and general sanitation by personnel (Althouse, 2008).
The extender-concentrates are diluted in distilled or de-ionized water. Next to the bacterial quality of the water, the electrolyte content, especially the absence of calcium ions, is an important characteristic for the water used to make the extender.
The comparison of different semen extenders has been subject of two kinds of studies: studies comparing different extenders in vitro, focusing on quality of semen after storage (De Ambrogi et al., 2006; Vyt et al., 2004a) and studies comparing fertility in vivo after insemination of semen stored for several days or stored in different extenders (Haugan al., 2007; Kuster and Althouse, 1999).
In vitro experiments showed no differences in cell viability between short-term and longterm extenders during 9-day storage (De Ambrogi et al., 2006). Motility remained unchanged within the first 72 hours, even in BTS-extenders, the most widely used shortterm extender. Based on in vivo results, differences were noticed between different extenders but it was not always possible to relate these differences to the type of extender. Differences between long-term extenders were observed from day 4 of storage onwards (Kuster and Althouse, 1999). The limited number of extenders compared in each study makes it difficult to set up a ranking of the semen preserving quality of long-term semen extenders.
4.3 Storage of porcine semen in frozen state
As mentioned above, porcine spermatozoa are particularly sensible to low temperatures and to rapid cooling due to the specific composition of the cell membrane (De Leeuw al., 1990). Cold shock can be solved technically by inducing cold resistance, namely incubating sperm at ambient temperature for several hours (Watson, 1995), by contact with seminal plasma that has a protective effect on spermatozoa (Centurion et al., 2006), together with controlled freezing protocols. The variation in freezability of individual boar’s semen is however more difficult to solve.
Semen extenders for frozen boar semen are completely different from extenders for liquid semen. The presence of egg-yolk, containing low density lipoproteins and cholesterol, has a protective effect on sperm membrane during cooling (Bathgate et al., 2006). Cryoprotectants, especially glycerol are added in low concentration to the medium in order to diminish membrane damage by freezing. Additionally, sugars and synthetic detergents are added, the latter having a modifying effect on the egg yolk inducing a better membrane stability of the cell membrane (Johnson et al., 2000). Thawing of the semen dose has been another point of concern. Thawing has to be fast in order to maintain sperm motility and acrosome integrity afterwards. Both processes i.e. freezing and thawing result in plasma membrane changes, explaining the variety of protocols available.
The fertility results with frozen semen have improved: cervical insemination results in a 75% farrowing rate and a litter size of 9.6 (Roca et al., 2003). Nevertheless, freezing and thawing are time consuming processes, restricting the use of frozen semen for specific indications, such as long transport times and conservation of valuable genetic material.
5. Insemination strategies
The management of AI is very important to determine the success of the procedure and the reproductive performance of the sows. Estrus control, timing and number of inseminations, the technique of AI, semen storage on farm and the use of new AI technologies, all require a specialized knowledge of pig reproductive physiology. The following measures could be taken to optimize the efficiency of AI on pig herds.
5.1 Boar stimuli
Correct timing of insemination requires careful detection of oestrus at regular intervals. Boar stimuli are important in promoting follicular development and expression of oestrus behaviour (Langendijk et al., 2006). Additionally, a high level of boar stimuli increases the frequency of uterine contractions, indicating a supportive role for passive sperm transport through the long uterine horns at the time of insemination. This effect can only be partially mimicked by a robot teaser boar which emits olfactory, acoustic and visual boar cues (Gerritsen al., 2005). Increase of oxytocin concentrations in peripheral blood plasma occurs in immediate response to boar presence and lasts for approximately 10 min (Langendijk et al., 2003). Therefore, exposure of sows to a boar during both back pressure testing and insemination is crucial.
5.2 Timing of insemination
Many studies have investigated time-relationships between oestrus, ovulation, insemination and fertilization using ultrasound testing. The key observation is that ovulation occurs at the beginning of the last third of oestrus regardless of the overall duration of oestrus. Precise prediction of the time of spontaneous ovulation in individual pigs has not yet been achieved. However, prediction of oestrus duration by observing the onset of oestrus after weaning has found broad acceptance in AI practice for calculation of the expected time of ovulation (Weitze et al., 1994). AI should be timed as close as possible to ovulation, preferably within 12 to 24 h before ovulation. The benefit of ultrasound testing of ovarian morphology for pig fertility management has been shown in practice (de Jong et al., 2009). Determination of the time of ovulation in relation to oestrus behaviour and AI management in representative numbers of sows on consecutive days has a great potential to provide short cuts in AI timing and to develop farm-specific strategies for improvement of AI management.
5.3 Use of new AI technologies
The development of techniques to inseminate with low numbers of spermatozoa in a small volume has increased insemination efficiency. This is particularly interesting when using spermatozoa of high value that are impaired, e.g. by freezing and thawing or sex-sorting. Post-cervical or intrauterine insemination with several devices has been developed to traverse the cervix and deposit sperm in the uterine body or posterior horn of multiparous sows. Compared to standard transcervical AI, post-cervical AI allows a threefold reduction in the numbers of spermatozoa to be inseminated, whereas deep intrauterine AI allows a 5 to 20 fold reduction (Vazquez et al., 2008). The use of post-cervical insemination varies among and within countries. Limits may arise from the use in sows only, skills needed for catheter handling, and the possibility of damaging cervical or uterine tissue. Semen encapsulation in a barium alginate membrane has been demonstrated to allow a single insemination (Vigo et al., 2009). Laparoscopy offers the possibility of inseminating a very low number of spermatozoa (i.e. 0.3 x 106) into the oviduct in anaesthetized pigs. However, the risk of polyspermic fertilization is substantial. Due to surgical intervention, its use is not appropriate in practice.
6. Conclusions
AI of swine is widely practiced and is a very useful tool to introduce superior genes into sow herds, with minimal risk for disease transmission. In practice, fresh diluted semen (3 billion spermatozoa in 80-100 ml) is mostly used for intracervical insemination .The success of AI is largely determined by the semen quality and the insemination procedure. Different parameters and techniques can be used to assess semen quality. Although more advanced technologies offer more accurate information, in commercial AI centres, semen quality is assessed based predominantly on concentration, morphology and motility using simple, cheap and practically easy-to-perform techniques. Critical issues for AI involve oestrus detection in the sow, timing of insemination and applying strict hygiene measures. Future developments will focus on new technologies to better assess semen quality in practice, to preserve semen for a longer time and to inseminate sows successfully using a lower number of spermatozoa using new AI techniques.
7. References
Alm, K.; Peltoniemi, O.; Koskinen, E. & Andersson, M. (2006). Porcine field fertility with two different insemination doses and the effect of sperm morphology. Reproduction in Domestic Animals, Vol.41, pp. 210-213, ISSN 0936-6768
Althouse, G. (2008). Sanitary procedures for the production of extended semen. Reproduction in Domestic Animals, Vol.43, pp. 374-378, ISSN 0936-6768
Althouse, G. & Hopkins, S. (1995). Assessment of boar sperm viability using a combination of two fluorophores. Theriogenology, Vol.43, pp. 595-603, ISSN 0093-691X
Althouse, G.; Kuster, C.; Clark, S. & Weisiger, R. (2000). Field investigations of bacterial contaminants and their effects on extended porcine semen. Theriogenology, Vol.53, pp. 1167-1176, ISSN 0093-691X
Althouse, G. & Lu, K. (2005). Bacteriospermia in extended porcine semen. Theriogenology, Vol.63, pp. 573-584, ISSN 0093-691X
Althouse, G.; Wilson, M.; Kuster, C. & Parsley, M. (1998). Characterisation of lower temperature storage limitations of fresh-extended porcine semen. Theriogenology, Vol.50, pp. 535-543, ISSN 0093-691X
Amann, R. (2009). Considerations in evaluating human spermatogenesis on the basis of total sperm per ejaculate. Journal of Andrology, Vol.30, pp. 626-641, ISSN 0196-3635
Barrabes Aneas, S.; Gary, B. & Bouvier, B. (2008). Collectis® automated boar collection technology. Theriogenology, Vol.70, pp. 1368–1373, ISSN 0093-691X
Bathgate, R.; Maxwell, W. & Evans, G. (2006). Studies on the effect of supplementing boar semen cryopreservation media with different avian egg yolk types on in vitro postthaw sperm quality. Reproduction in Domestic Animals, Vol.41, pp. 68-73, ISSN 0936- 6768
Boe-Hansen, G., Christensen, P., Vibjerg, D., Nielsen, M. & Hedeboe, A. (2008). Sperm chromatin structure integrity in liquid stored boar semen and its relationships with field fertility. Theriogenology, Vol.69, pp. 728-736, ISSN 0093-691X
Britt, J.H.; Almond, G.W. & Flowers, W.L. (1999). Diseases of the reproductive system. In: Straw B, D’Allaire S, Mengeling W, Taylor D (Editors), Diseases of Swine, 8th Edition, Blackwell Science Ltd, Oxford, pp. 903.
Camus, A.; Camugli, S.; Lévêque, C.; Schmitt, E. & Staub, C. (2011). Is photometry an accurate and reliable method to assess boar semen concentration?. Theriogenology, Vol.75, pp.577-583, ISSN 0093-691X
Centurion, F.; Echeverria, S.; Canul, N.; Ake, R.; Alfaro, M.; Santos, R. & Sarmiento, L. (2006). Influence of seminal plasma added to highly diluted semen on sperm motility of young boar feed with selenium and vitamin E. Reproduction in Domestic Animals, Vol.41, pp. 103, ISSN 0936-6768
Christensen, P.; Stryhn, H. & Hansen, C. (2005). Discrepancies in the determination of sperm concentration using Bürker-Türk, Thoma and Makler counting chambers. Theriogenology, Vol.63, pp. 992–1003, ISSN 0093-691X
Christensen, P.; Stenvang, J. & Godfrey, W. (2004). A flow cytometric method for rapid determination of sperm concentration and viability in mammalian and avian semen. Journal of Andrology, Vol.25, pp. 255-264, ISSN 0196-3635
De Ambrogi, M.; Ballester, J.; Saravia, F.; Caballero, I.; Johannisson, A.; Wallgren, M.; Andersson M. & Rodriguez-Martinez H. (2006). Effect of storage in short- and longterm commercial semen extenders on the motility, plasma membrane and chromatin integrity of boar spermatozoa. International Journal ofAndrology, Vol.29, pp. 43-552, ISSN 0196-3635
de Andrade, A.; de Arruda, R.; Celeghini, E.; Nascimento, J.; Martins, S.; Raphael, C. & Moretti, A. (2007). Fluorescent stain method for the simultaneous determination of mitochondrial potential and integrity of plasma and acrosomal membranes in boar sperm. Reproduction in Domestic Animals, Vol.42, pp. 190-194, ISSN 0936-6768
de Jong, E.; Vanderhaeghe, C.; Beek, J.; Van Soom, A.; de Kruif, A. & Maes, D. (2009). Ultrasonography of the ovaries in the sow: a helpful tool to determine the time of insemination. Vlaams Diergeneeskundig Tijdschrift, Vol.78, pp. 276-281, ISSN 0303- 9021
De Leeuw, F.; Colenbrander, B. & Verkleij, A. (1990). The role membrane damage plays in cold shock and freezing injury. Reproduction in Domestic Animals Supplement, Vol.1, pp. 95-104, ISSN 0936-6768
Donadeu, M. (2004). Advances in male swine artificial insemination (AI) techniques. The Pig Journal, Vol.54, pp. 110-122, ISSN 1352-9749
Ericsson, S.; Garner, D.; Thomas, C.; Downing, T. & Marshall, C. (1993). Interrelationships among fluorometric analyses of spermatozoal function, classical semen quality parameters and the fertility of frozen-thawed bovine spermatozoa. Theriogenology, Vol.39, 1009-1024, ISSN 0093-691X
Gadea, J. (2005). Sperm factors related to in vitro and in vivo porcine fertility. Theriogenology, Vol.63, pp. 431-444, ISSN 0093-691X
Gadea, J.; Sellés, E. & Marco, M. (2004). The predictive value of porcine seminal parameters on fertility outcome under commercial conditions. Reproduction in Domestic Animals, Vol.39, 303-308, ISSN 0936-6768
Gerrits, R.; Lunney, J.; Johnson, A.; Pursel, V.; Kraeling, R.; Rohrer, G. & Dobrinsky, J. (2005). Perspectives for artificial insemination and genomics to improve global swine populations. Theriogenology, Vol.63, 283–299, ISSN 0093-691X
Gerritsen, R.; Langendijk, P.; Soede, N.M. & Kemp, B. (2005). Effects of (artificial) boar stimuli on uterine activity in estrous sows. Theriogenology, Vol.64, pp. 1518-1525., ISSN 0093-691X
Green, C. & Watson R. (2002). Adverse effect of winter overnight temperature on transported liquid boar semen. The Veterinary Record, Vol.151, pp. 302-303, ISSN 0042-4900
Hànsen, C. & Hedeboe, A. (2003). Determining concentration of raw semen: test of the nucleocounter SP-100, MiniTüb SDM5 and Coring 254. DanskeSlagterier, Landsudvalget for Svin, report 613
Haugan, T.; Gaustad, A.; Reksen, O.; Gröhn, Y. & Hofmo, P. (2007). Fertility results of artificial inseminations performed with liquid boar semen stored in X-CellTMvs BTS Extender. Reproduction in Domestic Animals, Vol.42, pp. 94-99, ISSN 0936-6768
Henning, H.; Petrunkina, A.M.; Harrison, R.A. & Waberski, D. (2009). Changes in responsiveness to bicarbonate under capacitating conditions in liquid preserved boar spermatozoa in vitro. Society for Reproduction and Fertility Supplement, Vol.66, pp. 79-80, ISSN 1470-1626
Holt, C.; Holt, W. & Moore, H. (1996). Choice of operating conditions to minimize sperm subpopulation sampling bias in the assessment of boar semen by computer-assisted semen analysis. Journal of Andrology, Vol.17, pp. 587-596, ISSN 0196-3635
Holt, C.; Holt, W.; Moore, H.; Reed, H. & Curnock, R. (1997). Objectively measured boar sperm motility parameters correlate with the outcomes of on-farm inseminations: results of two fertility trials. Journal of Andrology, Vol.18, pp. 312-323, ISSN 0196- 3635
Holt, W.; Watson, P.; Curry, M. & Holt, C. (1994). Reproducibility of computer-aided semen analysis; comparison of five different systems used in a practical workshop. Fertility and Sterility, Vol.62, pp. 1277–1282, ISSN 1556-5653
Johnson, L.A.; Weitze, K.F.; Fiser, P. & Maxwell, W.M.C. (2000). Storage of boar semen. Animal Reproduction Science, Vol.62, pp. 143–172, ISSN 0378-4320
Knox, R. (2004). Practicalities and pitfalls of semen evaluation. Advances in Pork production, Vol.15, pp. 315-322, ISSN 1489-1395
Ko, J.; Evans, L. & Althouse, G. (1989). Toxicity effects of latex gloves on boar spermatozoa. Theriogenology, Vol.31, pp. 1159-1164, ISSN 0093-691X
Kommisrud, E.; Paulenz, H.; Sehested, E. & Grevle, I. (2002). Influence of boar and semen parameters on motility and acrosome integrity in liquid boar semen stored for five days. Acta Veterinaria Scandinavica, Vol.43, pp. 49–55, ISSN 0044-605X
Kondracki, S. (2003). Breed differences in semen characteristics of boars used in artificial insemination in Poland. Pig News and Information, Vol.24, pp. 119N-122N, ISSN 0143-9014
Kuster, C (2005). Sperm concentration determination between hemacytometric and CASA systems: Why they can be different? Theriogenology, Vol.64, pp. 614–617, ISSN 0093- 691X
Kuster, C.E. & Althouse, G.C. (1999). The fecundity of porcine semen stored for 2 to 6 days in AndrohepR and X-cellTM extenders. Theriogenology, Vol.52, pp. 365–376, ISSN 0093-691X0
Langendijk, P.; Bouwman, E.; Kidson, A.; Kirkwood, R.; Soede, N. & Kemp, B. (2002). Role of myometrial activity in sperm transport through the genital tract and in fertilization in sows. Reproduction, Vol.123, pp. 683-690, ISSN 1470-1626
Langendijk, P.; Bouwman, E.G.; Schams, D.; Soede, N.M. & Kemp, B. (2003). Effects of different sexual stimuli on oxytocin release, uterine activity and receptive behavior in estrous sows. Theriogenology, Vol.59, pp. 849-61, ISSN 0093-691X
Langendijk, P.; Soede, N.M. & Kemp, B. (2006). Effects of boar stimuli on the follicular phase and on oestrus behaviour in sows. Society for Reproduction and Fertility Supplement, Vol.62, pp. 219-30, ISSN 1470-1626
López, A.; Rijsselaere, T.; Van Soom, A.; Leroy, J.L.; De Clercq, J.B.; Bols, P.E. & Maes, D. (2010). Effect of organic selenium in the diet on sperm quality of boars. Reproduction in Domestic Animals, Vol.45, pp. 297-305, ISSN 0936-6768
López, A.; Rijsselaere, T.; Bijttebier, J.; Vyt, Ph.; Van Soom, A. & Maes, D. (2011). Effectiveness of the sperm quality analyzer (SQA-Vp) for porcine semen analysis. Theriogenology, Vol.75, pp. 972-977, ISSN 0093-691X
Maes, D.; Nauwynck, H.; Rijsselaere, T.; Mateusen, B.; Vyt, Ph.; de Kruif, A. & Van Soom, A. (2008). AI transmitted diseases in swine: an overview. Theriogenology, Vol.70, pp. 1337-1345, ISSN 0093-691X
Maes, D.; Rijsselaere T.; Vyt, Ph.; Sokolowska, A.; Deley, W. & Van Soom, A. (2010). Comparison of five different methods to assess the concentration of boar semen. Vlaams Diergeneeskundig Tijdschrift, Vol.79, pp. 42-48, ISSN 0303-9021
Mahmoud, A.; Depoorter, B.; Piens, N. & Comhaire, F. (1997). The performance of 10 different methods for the estimation of sperm concentration. Fertility and Sterility, Vol.68, pp. 340–345, ISSN 1556-5653
Martin-Rillo, S.; Martinez, E.; Garcia C.;Artiga C. & De Alba, C. (1996). Boar semen evaluation in practise. Reproduction in Domestic Animals, Vol.31, pp. 519-526, ISSN 0936-6768
Peña, F.; Saravia, F.; Garcia-Herreros, M.; Nunes-Martinez, I.; Tapia, J.; Johannisson, A.; Wallgren, M. & Rodriquez-Martinzez, H. (2005). Identification of sperm morphometric subpopulations in two different portions of the boar ejaculate and its relation to post-thaw quality. Journal of Andrology, Vol.26, pp. 716-723, ISSN 0196- 3635
Pérez-Llano, B.; Lorenzo, J.; Yenes, P.; Trejo, A. & Garcia-Casado, P. (2001). A short hypoosmotic swelling test for the prediction of boar sperm fertility. Theriogenology, Vol.56, pp. 387-398, ISSN 0093-691X
Petrunkina, A.; Gröpper, B.; Günzel-Apel, A. & Töpfer-Petersen, E. (2004). Functional significance of the cell volume for detecting sperm membrane changes and predicting freezability in dog semen. Reproduction, Vol.128, pp. 829-842, ISSN 1470- 1626
Popwell, J. & Flowers, W. (2004). Variability in relationships between semen quality and estimates of in vivo and in vitro fertility in boars. Animal Reproduction Science, Vol.81, pp. 97-113, ISSN 0378-4320
Rijsselaere, T.; Van Soom, A.; Maes, D. & de Kruif, A. (2002). Use of the Sperm Quality Analyzer (SQA II-C) for the assessment of dog sperm quality. Reproduction in Domestic Animals, Vol.37, pp. 158–163, ISSN 0936-6768
Rijsselaere, T.; Van Soom, A.; Maes, D. & de Kruif, A. (2003). Effect of technical settings on canine semen motility parameters measured by the Hamilton–Thorne analyzer. Theriogenology, Vol.60, pp. 1553–1568, ISSN 0093-691X
Rijsselaere, T.; Van Soom, A.; Tanghe, S.; Coryn, M.; Maes, D. & de Kruif, A. (2005). New techniques for the assessment of canine semen quality: A review. Theriogenology, Vol.64, pp. 706-719, ISSN 0093-691X
Roca, J.; Carvajal, G.; Lucas, X.; Vasquez, J. & Martinez, E. (2003). Fertility of weaned sows after deep intrauterine insemination with a reduced number of frozen-thawed spermatozoa. Theriogenology, Vol.60, pp. 77-87, ISSN 0093-691X
Shipley, C. (1999). Breeding soundness examination in the boar. Swine Health and Production, Vol.7, pp. 117–120, ISSN 1066-4963
Tomlinson, M.; Turner, J.; Powell, G. & Sakkas, D. (2001). One-step disposable chambers for sperm concentration and motility assessment: how do they compare with the World Health Organization's recommended methods? Human Reproduction, Vol.16, pp. 121-124, ISSN 0268-1161
Tsakmakidis, I.; Lymberopoulos, A. & Khalifa, T. (2010). Relationship between sperm quality traits and field-fertility of porcine semen. Journal of Veterinary Science, Vol.11, pp. 151-154, ISSN 1229-845X
Vazquez, J.M.; Roca, J.; Gil, M.A.; Cuello, C.; Parilla, I.; Vazquez, J.L. & Martínez, E.A. (2008). New developments in low-dose insemination technology. Theriogenology, Vol.70, pp. 1216-24, ISSN 0093-691X
Verstegen, J.; Iguer-ouada, M. & Onclin, K. (2002). Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology, Vol.57, pp. 149–179, ISSN 0093-691X
Vigo, D.; Faustini, M.; Villani, S.; Orsini, F.; Bucco, M.; Chlapanidas, T.; Conte, U.; Ellis, K. & Torre, M.L. (2009). Semen controlled-release capsules allow a single artificial insemination in sows. Theriogenology, Vol.72, pp. 439–444, ISSN 0093-691X
Vyt, Ph. (2007). Examination and storage of liquid porcine semen. PhD thesis, Ghent University, pp. 156, ISBN 978-90-586-4121-2
Vyt, Ph.; Maes, D.; Dejonckheere, E.; Castryck, F. & Van Soom, A. (2004a). Comparative study on five different commercial extenders for boar semen. Reproduction in Domestic Animals, Vol.39, pp. 1-5, ISSN 0936-6768
Vyt, Ph.; Maes, D.; Quinten, C.; Rijsselaere, T.; Deley, W.; Aarts, M.; de Kruif, A. & Van Soom, A. (2008). Detailed motility evaluation of boar semen and its predictive value for reproductive performance in sows. Vlaams Diergeneeskundig Tijdschrift, Vol.77, pp. 291-299, ISSN 0303-9021
Vyt, Ph.; Maes, D.; Rijsselaere, T.; Dejonckheere, E.; Castryck, F. & Van Soom, A. (2004b). Motility assessment of porcine spermatozoa: a comparison of methods. Reproduction in Domestic Animals, Vol.39, pp. 447-453, ISSN 0936-6768
Vyt, Ph.; Maes, D.; Rijsselaere, T.; Dewulf, J.; de Kruif, A. & Van Soom, A. (2007). Semen handling in porcine artificial insemination centres: the Belgian situation. Vlaams Diergeneeskundig Tijdschrift, Vol.76, pp. 195-200, ISSN 0303-9021
Waberski, D. (2009). Critical steps from semen collection to insemination. Proceedings of the Annual Meeting of the EU-AI-Vets, September 2009, Gent Belgium, pp. 66-69
Waberski, D.; Petrunkina A. & Töpfer-Petersen E. (2008). Can external quality control improve pig AI efficiency? Theriogenology, Vol.70, pp. 1346-1351, ISSN 0093-691X
Waberski, D., Schapmann, E., Henning, H., Riesenbeck, A. & Brandt, H. (2011). Sperm chromatin structural integrity in normospermic boars is not related to semen storage and fertility after routine AI. Theriogenology, Vol.75, pp. 337-345, ISSN 0093- 691X
Watson, P. (1995). Cooling of spermatozoa and fertilizing capacity. Reproduction in Domestic Animals, Vol.31, pp. 135-140, ISSN 0936-6768
Weitze, K. (1990). The use of long-term extenders in pig AI - a view of the international situation. Pig News and Information, Vol.11, pp. 23-26, ISSN 0143-9014
Weitze, K.F.; Wagner-Rietschel, H.; Waberski, D.; Richter, L. & Krieter, J. (1994). The onset of oestrus after weaning, heat duration, and ovulation as major factors in AI timing in sows. Reproduction in Domestic Animals, Vol.29, pp. 433-443, ISSN 0936-6768
Wilson-Leedy, J. & Ingermann, R. (2007). Development of a novel CASA system based on open source software for characterization of zebrafish sperm motility parameters. Theriogenology, Vol.67, pp. 661-672, ISSN 0093-691X
Woelders, H. (1991). Overview of in vitro methods for evaluation of semen quality, Proceedings of the second International Conference on Boar Semen Preservation, KF Weitze and B Colenbrander (Editors), Paul Parey Scientific Publishers, Berlin and Hamberg, pp. 145-165
Xu, X.; Pommier, S.; Arbov, T.; Hutchings, B.; Sotto, W. & Foxcroft, G. (1998). In vitro maturation and fertilization techniques for assessment of semen quality and boar fertility. Journal of Animal Science, Vol.76, pp. 3079-3089, ISSN 0021-8812
Related topics:
Authors:
Ghent University
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.






