Introduction
The prevention of FHB on the field becomes highly important. The toxin is typically of field origin. In the postharvest period and storage further increase of toxins may occur, but the field prevention is the key. The breeding approach becomes more important as there are now three regulations for toxin contamination for Fusarium toxins. The Commission Regulations, EC 856/2005 (2005) and EC 1126/2007 (2007) set limits for contamination of basic materials for human food and food products. For animal feeds, the Commission Regulation EC 576/2006 (2006) gives suggested values. Epidemics cause serious yield losses, however in most of cases the toxin contamination is crucial as this makes the harvested yield unsuitable for any later uses. Therefore the reduction of toxins becomes inevitable in cereal commodities.
In this paper two means will be treated, the possibilities of the breeding and the fungicide application. Both topics have been discussed many times in the last decades, but fields are still ruled mostly by susceptible and highly susceptible cultivars exposed to the epidemics when conditions favor disease development. It means that growing resistant varieties remained a dream for the farmers.
Breeding for resistance. The breeding is a real green solution; food safety can be ensured without any pesticide use. However, at present most varieties are susceptible or highly susceptible as this trait was not considered during the selection of parents exposed to epidemics. An additional problem is that natural infection pressure is usually not severe enough to have sufficient selection pressure; therefore most of the cultivars will be susceptible. For this reason artificial inoculation systems should be introduced to have the necessary selection pressure for identifying the superior genotypes. The breeding issues have been summarized several times in the last decades (Dill-Macky 2003, Gilbert & Tekauz 2000, Mesterházy 1995, 2003, Miedaner 1997, Parry et al., 1995, Snijders 2004). Effective resistance sources were identified, inoculation techniques were developed for different scientific and practical approaches (Dill-Macky 2003), and large amounts of materials were screened to detect variability in native populations.
There were several questions we had to find answers for. The FHB is caused by many Fusarium spp., in Hungary we identified 16 (Mesterházy 1984), and many other sources report similar species diversity (Sommer et al., 2008, Stepien et al., 2008, Mankeviciene et al., 2008). For this reason it was important whether a common resistance to different Fusarium spp. exists or not. According to Mesterházy (1987, 2002, 2003) and Mesterházy et al. 2005) the resistance protects against all Fusarium spp. tested, this means that selection against a single pathogenic isolate is effective to all Fusarium spp. This is true also for the members of the F. graminearum species complex (Tóth et al. 2008). Earlier sources clarified that within F. graminearum no vertical races exist, this is in agreement with these novel findings (Miedaner 1997, Snijders 1994). The repeatability of the used methods was good (Mesterházy 1995, Mesterházy et al. 1999, 2005), so a breeding application of the methodology was justified. Looking at the symptoms to be considered we concentrated on the relations between disease symptoms and DON contamination. The results clearly showed that the ratio of Fusarium damaged kernels (FDK) presented closer and more reliable relations with DON than visual assessment and other traits measured. This has several well documented reasons. The conclusion is that the central trait is FDK; the visual head assessment is suitable only for preliminary selection. For this reason, in the selection process we select consequently to low disease severity in visual symptoms and thereafter for FDK. DON will be checked normally only for the low infection severity samples. Therefore in this paper we present yearly data for the selection material. As they change for year to year, the experiment will be checked by the closeness of the correlation between FHB and FDK values, which is normally cca. r = 0.80-, and the performance of the control cultivars that are sown and inoculated in several replicates in different parts of the experiments.
According to general field experience the fungicide efficacy remained low in epidemic years, the mean was not better than 20-30 % reduction of disease severity or toxin contamination. At this reduction level natural epidemics producing higher than 1.5-1.7 mgDON cannot be properly controlled. This means we remain without protection when it would be the highest necessity. For a long time fungicides were blamed and more and more effective fungicides were requested from the producers. However, the truth is more complex. Mesterházy and Bartók (1996) , Mesterházy et al. (2003a, 2003b) and Mesterházy et al. (2003) proved that fungicide efficacy at optimal coverage of heads by fungicide spray may reach 80-90 % reduction for the most effective fungicides. The correlations between the reactions of traits like FHB severity, FDK and DON were very close, up to r = 0.90 in most experiments, so the different traits were similarly reduced. At a level of 80 % of reduction the reduction from 5 mgto 1 mgcan be secured, at 90 % reduction the epidemics up to 10 mgDON concentrations can be controlled. The reasons are as followed: (1) Coverage. Our tests were made at optimal coverage with hand sprayed treatments from both sides to cover the whole ear uniformly. McMullen et al. (1997, 1999) have shown first that spraying technology significantly influences fungicide efficacy. Hooker and Schaafsma (1984) compared the coverage of heads at different nozzle types and they concluded that nozzles traditionally developed for leaf control give poor coverage, and so poor efficacy. Ruden et al., (2005) found that a deep penetration of the spray down to the rachis is an important condition to have effective protection. (2) Translocation. The problem is that fungicides are only partly systemic, they move acropetally, so from the leaves there is no way to the heads (Mauler- Machnik and Zahn, 1994). We tested this phenomenon in the seventies in 48 wheat genotypes (Mesterházy unpublished). The fungicide spraying was made at boot stage, the check received no fungicide treatment. At flowering both versions were artificially inoculated by four isolates of Fusarium. The general means were equal, but a variation in reaction of individual genotypes was observed. This supported also the view of the lack of translocation. For this reason the fungicide should be placed on the ear directly from every side, only this can secure an effective protection. (3) Fungicide differences. In the past 20 years a large number a fungicides were tested. Our ranking corresponded well with that of Maufras et al. (1994), but with much higher efficacy levels. (4) Variety resistance differences. Cultivars with higher resistance could be protected generally more effectively; the level of the toxin contamination was lower. The most sensitive cultivars could not be protected under the most heavy infection pressure by any of the fungicides; therefore this cultivar group should be withdrawn from commercial production.
The difference between practical farm data and our much higher efficacy data were termed as technology gap (Mesterházy et al. 2003). In this paper we summarize a three years study where we compared large scale farm application and hand made small pot tests in 2006- 2008. The question was how to come closer with efficacy on farm level to the finding of the small plot results and see, to what extent the gap could be bridged.
Materials and Methods
Breeding trials. The breeding program started with the selection of more resistant lines from winter wheat materials. In the program local more resistant native lines and Sumai- 3 and Nobeoka Bozu spring wheat resistance sources were used. The breeding followed the scheme reported by Mesterházy 1995, 2002, Mesterházy et al. 1999, 2005). The essence is that resistance breeding needs more resistant parents and a consequent screening technique in each generation to identify superior genotypes.
The isolates, their origin, the way of increase and testing of aggressiveness were published earlier (Mesterházy 1995, 2002, Mesterházy et al. 2005); therefore a detailed description is not given here. The inocula, like in all other tests in this paper were sprayed at full flowering with a 2.0 l hand sprayer (Eva, DiMartino, Mussolente, Italy) mediated by ear pressure. In these tests FHB severity was assessed five times, their mean was used as mean severity. In order to gain comparable data a careful threshing of the infected heads with no wind and air separation was made to keep all infected grains as at normal wind speed the light Fusarium infected grains are blown out. The fact that this rule is often not kept explains many low correlations between FHB, FDK and toxin contamination (Wilcoxson 1996).
Resistant lines, resistance sources and other materials being important for the selection as well as susceptible control varieties, lines or unknown materials received for resistance tests were sown in three row plots of 1.5 long and inoculated each year by four different Fusarium isolates (two F. graminearum No. 12377 and 46 and two F. culmorum No. 12375 and 12551) with different aggressiveness. It means that regarding the resistance level their means were much more informative than the results for only one single isolate. As there are no races and the resistance is the same against different Fusarium spp., the more precise resistance evaluation requests the use of more parallel isolates. In the case of failure of an isolate in the given test, the others may provide the necessary data, so the chance is to receive useful data is higher. Here besides FHB severity and FDK also DON contamination was recorded. In a year 100-150 lines are tested, in the first part of the results these results will be mentioned first. The second part of the breeding chapter is the resistance test of the variety candidates from the official variety registration trial by the authority. Yearly more than hundred lines were tested. The isolates were: two inocula from 12551 and one from 12375 F. culmorum, and 46.06 from F. graminearum. As the material changes very much from year by year, here the variability will be shown, but as the same control cultivar were inoculated in different parts of the trial, the repeatability of the data can also be followed in this way. In these trials two plots were sown, each plot consisted of six rows and four isolates were used in two groups of heads. The data from 2007 and 2008 are shown.
A third experiment was made with registered cultivars from medium to high susceptibility from 2006 to 2008 to see the differences and the repeatability. The tests followed the design outlined for the variety registration tests, the isolates used were F. culmorum 12375 and 12551 as well as F. graminearum 44/ J5A II and 12377; they were constant in the four years of the test. For DON analysis see the fungicide part below. From this only the DON values are presented in this paper.
Fungicide trials (2006-2008).
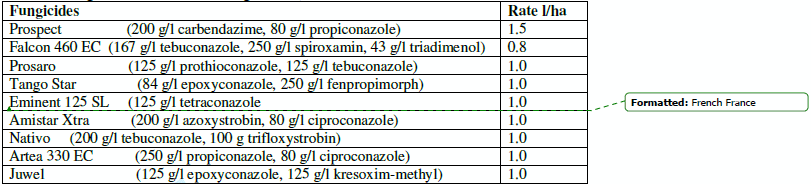
Small plot tests. The plot size was 5 m2. Three cultivars with different resistance to FHB were used, Petur (MR), Miska (S) and Kapos (MS). There were 3 replicates meaning that each fungicide treatment was represented by 9 plots. Heads were sprayed at full flowering with 2.0 l hand sprayer (Eva, DiMartino, Mussolente, Italy) mediated by air pressure. At spraying half of the 200 ml/plot was given in the front, the other half on the rear side to reach optimal coverage of spray. The fungicides and rates are shown in Table 1. Two days after the preventive fungicide treatment artificial inoculation was used as described by Mesterházy et al (2003). In each plot four isolates were used and each isolate was represented by three groups of heads. Additionally three groups of heads were used without inoculation for controls. Evaluations of disease, harvest, threshing and cleaning were made according to that described in the breeding part before and by Mesterházy et al. (2003).
Field applications. The same cultivars were sown in three ha field, altogether 9 ha. The sowing was made as it is used in seed production fields, between 1.2 m stripes 30 cm were left free to care plants, take data without damaging the stand. Fungicides and dose rates were the same as above. As flowering time was very similar (1-2 days difference) the whole test was sprayed the same day in full flowering. The spray volume was 250 l/ha, speed of the tractor was 7-8 km/h. The boom was 18 m, the left and right sides mounted with different type nozzles. The sprayed stripe was 7 m wide (five stripes) and 200 m long. As 10 treatments were made, the means of the fungicides and cultivars gave enough data to compare the efficacy of the nozzles, and the six plots for a fungicide treatment gave comparable picture about the performance of the fungicides. Additional replicates were not used. No artificial inoculation was used. In 2006 and 2008 more epidemic conditions ruled, but not more than 5- 7 % of the heads received infections (incidence) in the not protected control plots. Here the diseased part of the infected heads seldom was higher than 30 %. In 2007 the flowering time was still wet, but right thereafter a long dry period followed until harvest, so the starting epidemic ceased. Two nozzle types were used, the traditional TeeJet XR and the Turbo FloodJet, this latter was mounted on the boom at 50 cm distance, back and forward, so the could cover the heads from each side. As the angle of the spray jets was cca. 160° , the heads received spray from every side, ensuring a more optimal coverage close to the hand sprayed versions. Evaluation was made 15 and 22 days after fungicide treatment where the incidence was given as infected heads/m2. At harvest yield was measured, from each plot a sample of 1 kg grain separated for FDK and DON analyses.
DON analysis. The yields of three parallels of each isolate were pooled, these samples of six grams were milled and analyzed by HPLC (HP 1090M equipped with diode array detector, Hewlett Packard, now Agilent, USA). Details of extraction and analysis were reported in Mesterházy et al. (2003).
Statistical analysis. The non-replicated screening tests could not be statistically analyzed. The screening of resistant sources inoculated with four different isolates without replicates were evaluated by the two-way ANOVA model without replication. This model was used also for the DON evaluation of the third experiment with registered cultivars, where three years and four isolates were used. However, the grains of the replicates were pooled, therefore 12 data were available. This neutralized to some extent the large aggressiveness differences between isolates and years used. Except the early generation tests, randomized block design was used, for this as well as the correlation and regression analyses the Excel programs (Microsoft Inc.) were used. For the three-way variance analyses also the Excel was used, but with the functions given by Sváb 1973 (1973) and Weber (1967). In the farm-scale fungicide tests the SAS statistical program gave help.
Results
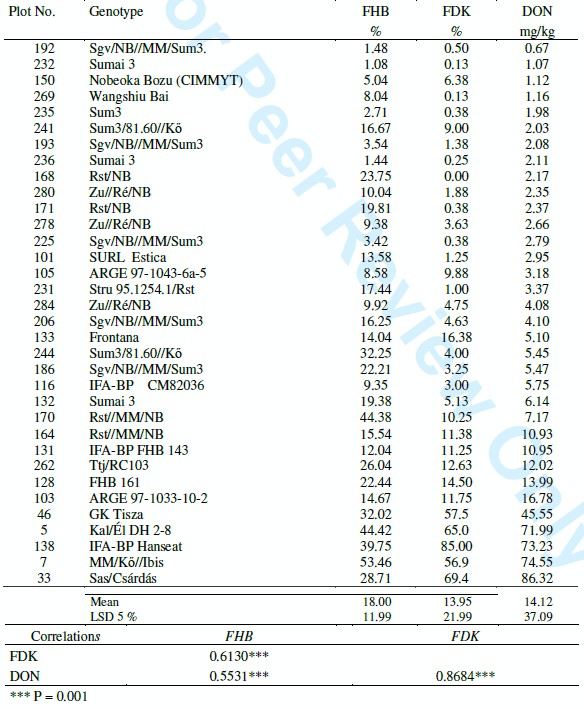
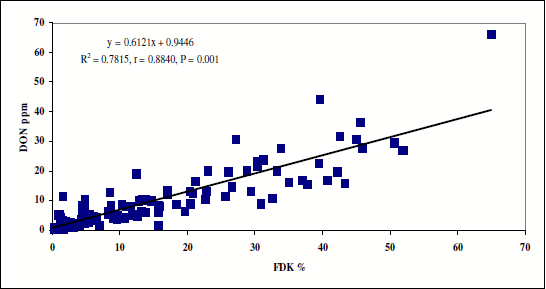
Pre-breeding lines and resistance sources. In 2005 117 lines from the breeding program were tested for FHB resistance against the four isolates of Fusarium. The mean data of FDK and DON are plotted on Figure 1. It is clear that the two traits closely interrelate. The most important toxin regulating agent is the resistance level. We see a similar picture from 2006 from 139 lines with the same result, the correlation coefficient between FDK and DON contamination is r = 0.8683, significant at P = 0.001. The only difference is that 2006 was epidemic, so higher DON values were achieved. These close correlations have significance also for the selection. In Table 2 a selection of the 139 genotypes is shown, the most resistant materials and several from the most susceptible ones are at the end of the list. Only those were selected that gave similar results in the previous years. The highly resistant and popular resistance sources like Sumai 3 have several accessions and there are slight differences between them. The DON differences are striking; from near zero up to 80 mgeverything occurs.. RSt/Nobeoka Bozu (NB) is winter wheat with much better agronomy type than NB was itself. Zu//Re/NB is a second generation material with improved agronomy and Ttj/RC103 that does not contain spring parents and has a fairly good resistance level. The Arge lines belong also to this group. At the end of the table highly susceptible genotypes take place.
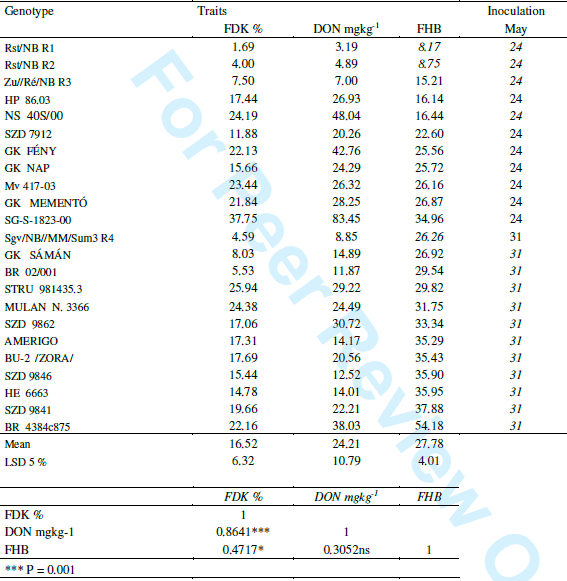
Results of the variety candidates and released cultivars. In 2006 only the 3rd year variety candidates were tested. They were supplemented with four resistant Szeged lines from the breeding program. The data are shown in Table 3.
We see more than ten fold difference between genotypes. The Szeged control lines have the best values, but the most resistant candidate had 11 mgDON, the most susceptible had 83 mg. This means about nine fold differences between candidate lines. The correlation coefficients show the same picture we have seen in Table 1, much closer correlation between FDK and DON than with data of FHB severity. GK Saman, a variety candidate from our program had good performance, but because of its high sensitivity to Septoria tritici the yielding ability was low and so it was not registered.
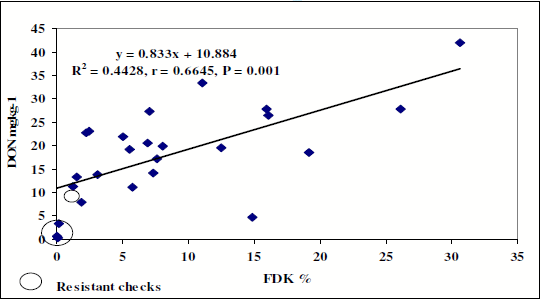
In 2007 the flowering period was wet, but thereafter the season was extremely dry and hot. The DON level was less (Maximum = 41 mg), but genotype differences were large and highly significant. (Figure 2).
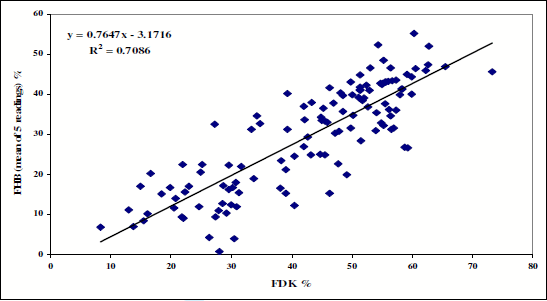
In 2008 125 variety candidates were tested (Figure 3). The differences between genotypes are striking again. The close correlation between FHB and FDK values corresponds to the results presented until now. For DON only the 3rd year candidates were tested (Figure 4) and presented with the FHB and FDK data. The DON contamination correlates with FHB and FDK data at r = 0.66 (P = 0.01). We found excellent DON producers like Quebon, and the Mv 19-05 candidate had three times less DON at the same FDK level The DON/FDK ratio varies between 1.21 and 3.95 indicating that 1 % of FDK may mean highly different DON data. The DON mean data across the four isolates used varied, the means were for the isolates 17.7, 97.1, 106.7 and 126.9 mg. The lowest and highest value was 0.31 and 394 mgDON.
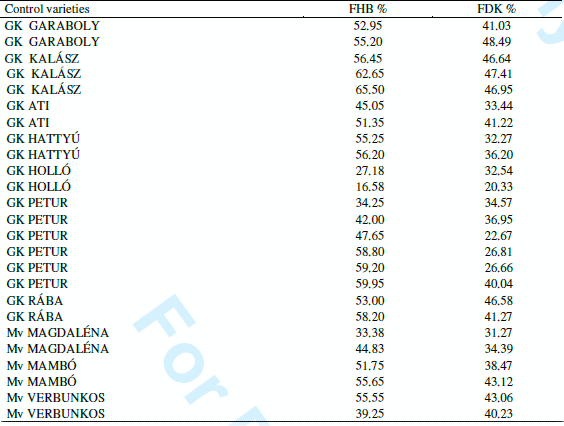
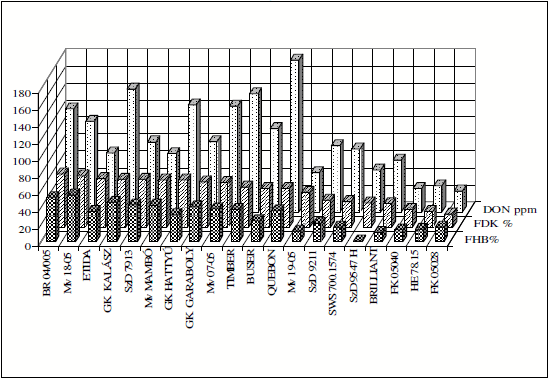
As in these tests the controls were sown several times across the nursery, their performance gives an idea about the repeatability of the results. The data (Table 4) clearly show that except Petur the other cvs gave very close results. So the repeatability within the test was good. The 28 registered cultivars showed large variability for DON contamination (Figure 5). There were four fold differences recorded as a mean across isolates and years. This shows again that among the existing cultivars a considerable variation was recorded. Therefore a selection of less susceptible cultivars is possible by the simple preference of the more resistant genotypes.
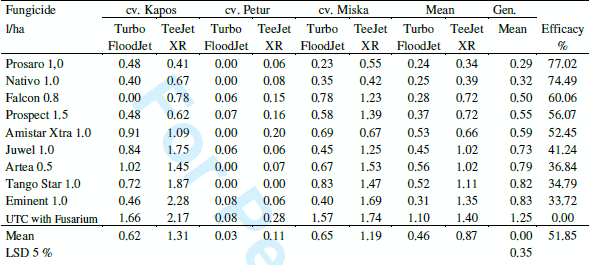
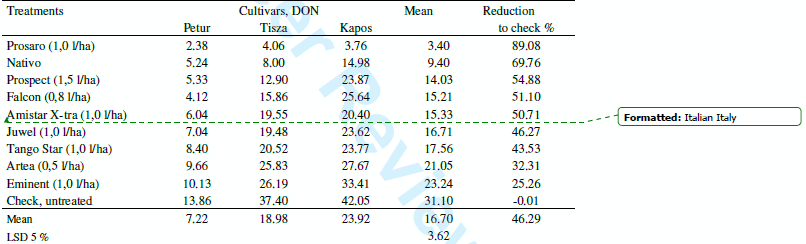
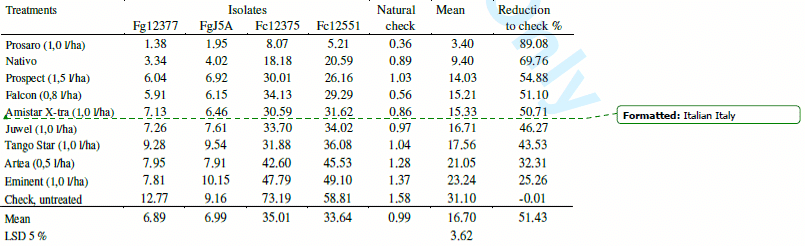
Fungicide tests. The three years data were evaluated for all FHB traits etc., however in this paper we concentrate only on the reduction of the DON contamination. In the small plot tests 1350 DON analyses were made during the three years. This seems to be a rather solid data base to draw well-supported conclusions. Table 5 shows the means for the three cultivars. The general tendencies are the same; even though the resistance level of the three cvs was different. The correlations between fungicide reactions of the three cultivars are between r = 0.87 and 0.94 (P=0.1 %) indicating that cultivars generally behave in a very similar manner. The mean efficacy of the fungicides is between 25 and 90 % at optimal coverage. The results were grouped also according to the isolates used (Table 6). The correlations between fungicide responses to the different isolates ranks from r = 0.78 to r = 0.95 indicating a very similar response at different epidemic situations. The reduction of the DON contamination is very similar with some deviations at the different epidemic severities. It is important that the protected, but artificially not inoculated control groups at the end of the plots presented similar ranking (natural infection) the correlations are all above r = 0.80 (P = 0.1 %) with the data of the fungicide protected and inoculated groups of heads. This agreement between data shows clearly that the fungicides really do their work, should it be evaluated under artificial or natural conditions.
Parallel with these tests the large scale farm application was also carried out. The question was how much of the efficacy reached in the small plot trials can be realized in the field. In this experiment two nozzle types were tested. The Turbo FloodJet that sprays nearly horizontal and so can covers the heads from side with much higher coverage than the TeeJet XR nozzle does that is used to protect the leaves and less the heads. The mean data (Table 7) according to cultivars and nozzle types show clear differences between fungicides and also nozzle types. The mean reduction of DON contamination was about 50 %, but less at the most effective fungicides, the mean for TeeJet XR is 0.87, and for the Turbo FloodJet nozzle 0.46 mgDON, a reduction of 47 % .. Field efficacies were somewhat lower than those of the hand made test with more precise coverage, however, they were closer than expected. For the best fungicide it was 77 % here and 89 in the small plot test. The mean efficacy was the same, but the best ones had higher and the least effective ones had lower numbers in the small plot tests.
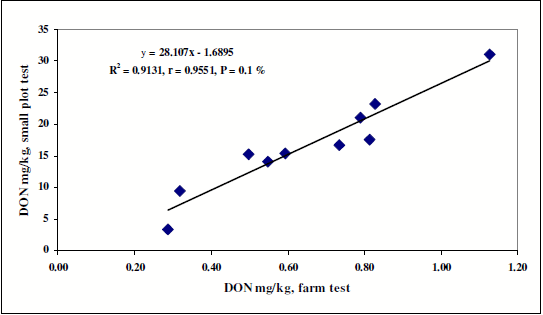
In the last figure (Figure 6) data from the hand made small plot tests and the large scale farm application are compared. The agreement is very good; the performance of the fungicides is very similar in the two application forms.
Discussion
Breeding and resistance screening. Many authors reported about smaller and larger resistance differences in native materials (Snijders 1990, Brown-Gudeira et al. 2008, Scott 1927, Christensen, et al., 1929, Blomquist and Jamalainen 1968, Leiteritz and Focke 1977, Capetti and Pirvu 1978, Lemmens et al., (2003), Polisenska and Tvaruzek (2007) and Miedaner et al., (2003) and Paul et al. (2005). We have tested large amount of materials from different sources and several good genotypes with medium resistance could be identified. Several crosses from the French Renan gave also better than medium resistance. Viginta from Slovakia belongs to this category. Good resistance was also detected in some land races from all over the world (Snijders 1990). These data show clearly that a variability exist in each nursery that could be utilized. It is true that most of the genotypes are susceptible, but not all and not at the same level. Zhou (1985) analyzed Chinese wheat breeding programs and much transgressive segregation was identified. The winter wheat breeder can have the conviction when they cross their better materials in a breeding program, they can receive plants with significantly higher resistance than any of the parental lines. Sumai 3 is an excellent example for this. The domestication of highly resistant agronomically poor resistance sources is surely important. The pre-breeding can provide the breeders new useful sources. It is not a problem. The data additionally show that the pre-breeding exotic sources was successful and considerable variation was found and exploited from local more resistant genotypes. It is important that many of these lines are agronomically good; some of them have the necessary quality and yielding ability . This is significant as breeding was normally thought to bring results, e.g. a new variety in 10-20 years. As our breeding program started more than 20 years ago, including the prebreeding, the first variety candidate was given to the state registration trials in 2009, and the suitable lines in the next years. It is remarkable that some genotypes have medium FHB severity, but very low FDK and DON contamination. We have also genotypes where FHB is 32 % at 5 mg DON and another with 28 % FHB and 86 mg DON contamination. The correlations show this well, the FDK has much closer correlation with FDK than with FHB severity. The difference is not always so large, but the tendency with the exception of 1-2 tests is the same. The repeatability of test results was good under different ecological conditions. Highly susceptible genotypes gave very variable results in different environments, however, resistant materials performed normally at much lower infection severity (Mesterházy et al. 1999) and more severe symptoms occurred only exceptionally at very severe infection pressure. The existing methodical background is suitable for breeding and also resistance tests, the repeatability of the test results was good as was show here and in earlier tests (Mesterházy 1995, Mesterházy et al. 199, 2005).
This study series convinced us, however, that a significant improvement of food safety can be achieved when the existing variability is exploited. Maybe that this resistance level is not as high that can be achieved in many years from now by breeding, but screening the advanced materials can improve the food safety by about 50 % or more, e. g. the toxin level can be decreased by this rate. As every breeding program has variability, for them the screening of their own material is important. As most breeding firms do not have any screening with artificial inoculation for this trait, the relevance of this activity is clear. The high variability among variety candidates allows the preference of the more resistant candidates. The data show clearly, that this is possible; it is not an accident that in China, Germany and the Netherlands the FHB tests are integrant parts of the registration process. When the registration process is going well, the rate of medium resistant and moderately susceptible genotypes will cover the major part of the wheat acreage. The screening results of the commercial varieties show again a possibility to increase food safety as the withdrawal of susceptible and highly susceptible cultivars from commercial production may also help a lot. Farmer associations and other organizations do such work like ITCF in France and publish their result regularly. It is important that the regular FHB screening for registration and commercial cultivars will have its feedback for the breeding and the exploitation of the existing variability in the breeding material and starting the breeding program with long term effects will be primary interest of the breeding firm to keep market.
In the last decades most breeding program considered the visual field symptoms. As the toxin limits were introduced, the main trait is the toxin contamination. The presented data clearly show that FDK correlates more closely with DON than visual field scores do. It is also not an accident. Having been finished the visual evaluation 25-30 days after flowering, we have 3-4 weeks until harvest. Late rains may induce significant additional disease development. According to our experience about 50 % of the genotypes with acceptable FHB severity should have been discarded. As DON and FDK correlate well, a toxin control during the breeding is not necessary. It is enough to select for low FDK level and then the best materials are to be tested also for DON before they submitted for registration to the authority.. It seems that the spraying inoculation covers a much more wide resistance background that the point inoculation does (Mesterházy et al. 2007). Therefore, for breeding and screening purposes a variant of the spraying inoculation or the spawn method should be used.
Fungicide application. The time span for a variety registration and the introduction of artificial inoculation methods is considerable, even it is much shorter than the breeding process. Considering the existing cultivars, 2-3 years would be enough to decide about them, so only thereafter the resistant ones can be favored. When a candidate will start the three years test period and then gets registered, it will need two additional years to produce enough seed to enter the market with it. This means minimum five years. This time span must be over bridged with fungicide use in European agriculture and other continents where the yields are high enough to make fungicide application economic. It is not new in these regions, only the efficacy is low. These results prove, however, that efficacy can be increased significantly. With increased resistance the fungicides can secure the necessary food safety, so the use of fungicides will not cease after the introduction of more resistant cultivars. We are sure that official toxin regulations will be stricter when production can keep the official limits, because immune suppressive influence of DON is significant at even at much lower concentration than allowed nod (Berek et al. 2001).
The fungicide results of the small plot trials fully support the statements of our earlier report (Mesterházy et al. 2003). The efficacy of the best fungicides is 80 % or higher for DON. We have two major conclusions. There are highly significant differences among fungicides regarding their efficacy varying between 25 and 90 %, a roughly four fold difference. Ninety % efficacy means that a natural epidemic until 10 mgkg-1 DON concentration can be managed. 25 % means that no effective protection can be hoped from these fungicides. For this reason it would be highly desirable to rate the fungicides like the ITCF in France does (Maufras et al. 1994). The fungicide ranking is similar, “only” the efficacy is much higher. The idea to have optimal coverage of the spray on the heads worked. These data are coming back in the past 20 years (Mesterházy et al. 1996, 2003). The reasons are also clear, why the better coverage is important. Beyond this the methodology is ready to determine the real anti- Fusarium activity of the fungicides. This excludes outdated application technology and other application and timing problems etc. The question is now, how can we develop the field application to come near to these values.
The large scale tests had the objective to see the practical realization of the innovation of the small plot experiments. The results are clear in two respects. Traditional XR nozzle gave as average 100 % higher DON contamination than the Turbo FloodJet nozzles. The fungicide ranking was similar for both nozzle types. There were, however, exceptions. Eminent responded much better in field application to the Turbo FloodJet nozzles, but the difference between reactions to the nozzles of Amistar Xtra was only very small. The others gave corresponding results. As we have results for three years, some solid reasons should be behind the results, but at present no clear explanation exists. We should therefore state that exceptions of any reason occur and might influence fungicide choice.
The variety resistance influences fungicide reaction. The uniform or uneven flowering is important. The best ones are those varieties having all tillers flower within 2-3 days. A longer flowering time is problematic as an early spray leaves the later coming heads unprotected, whereas a late spraying allows severe infection when the rains come at early flowering and these will be unprotected. Earlier cultivars are better as the 3-4 week protecting power is enough until harvest, for the cultivars with very long vegetation period this stage 3- 4 weeks are too short, and a later rainy period can cause significant damage. We observes also an interesting phenomenon, thus is the receptivity of the fungicide as a cultivar trait meaning the varieties with the same resistance level may respond differently to fungicides, one gives much better results than the other (Mesterházy 2010). The reasons are not known, but when developing a variety specific technology we should also consider these effects, moreover, experimental background will be needed to determine specific variety reactions.
The coverage was also controlled (data were not shown). Turbo FloodJet gave much better coverage on the front side of the sprayed heads, but the rear was covered much less as we anticipated based on Hooker and Schaafsma (2004). For this reason further research will be needed to find the nozzle type and its proper application to provide an even better coverage.
We have several important conclusions. Without effective fungicides no effective control is possible, even the most sophisticated technology cannot help. The efficacy differences between fungicides are striking and give an about 3-4 fold difference. We could verify the significance of the nozzle types, generally it reduced the DON contamination by an average of nearly 50 %, but at the most effective fungicide this ratio was smaller, about 30 %. The field application convinced us that the generally poor farm results are not only due to the use of traditional nozzles like TeeJet XR. The rare excellent results in the literature support this view (like McMullen et al. 1999). Lots of other technological errors could contribute to the often discouraging results. For this reason all effects should be considered when a fungicide program is planned, excellent fungicide, the best nozzle type and other important technology steps like timing, spray volume, speed of application, wind speed and others.
Acknowledgements. The Authors wish to express their thanks to NKTH-KPI for financial support via projects OMFB 01286/2004 and OMFB 00313/2006 as well as EC KBBE-2007- 222690-2 MYCORED.
References
Commission Regulation (EC) No 856/2005 of 6 June 2005, Official Journal of European Union 07.06.2005, L 143/3.
Commission Recommendation (EC) No. 2006/576 of 17 August 2006 on the presence of deoxynivalenol, zearalenone, ochratoxin A, T-2 and HT-2 and fumonisins in products intended for animal feeding (Text with EEA relevance). Official Journal of European Union 23.08.2006, L 229/7
Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Official Journal of European Union 29.09.2007, L 255/14
Berek L, Perti IB, Mesterházy Á, Téren J, Molnár J. 2001. Effect of mycotoxins on human immune functions in vitro. Toxicology in vitro. 15:25-30.
Blomqust HH, Jamalainen EA. 1968. Preliminary rest of winter wheat cereals of resistance to low temperature parasitic fungi in controlled conditions. Maataloust. Aikakakusk. 40:88-95.
Brown-Gudeira G, Griffey C, Kolb F, McKendry A, Murphy JP, van Sanford D. 2008. Breeding FHB-resistant soft winter wheat: progress and prospects. Cereal Research Communications 36, Suppl. B,. 31-36.
Capetti E, Pirvu T. 1978. Investigations on the resistance of some varieties and lines of winter wheat to Fusarium roseum f. sp. cerealis var. “Graminearum” (in Romanian). Lucrari Siintifice A. 18-19:7-17.
Christensen JJ, Stakman EC, and Immer, FR. 1929. Susceptibility of wheat varieties and hybrids to fusarial head blight in Minnesota. Univ. Minn. Agr. Exp. Sta., Techn. Bull. 59:1-23.
Dill-Macky R. 2003. Inoculation methods and evaluation of Fusarium head blight resistance in wheat. In: Leonard KJ. and Bushnell WR. (Eds) Fusarium head blight of wheat and barley. APS Press, St. Paul MN, USA. pp. 184-210.
Gilbert J. Tekauz A. 2000. Review: Recent developments in research on Fusarium head blight of wheat in Canada. Canadian Journal of Plant Pathology 22:1-8.
Hooker DC, Schaafsma AF. 2004. Effective application of fungicides on wheat heads: what’s the best? (Fungicide application systems for controlling Fusarium head blight). In: Canty SM, Boring T,Verasdahl K, Wardwell J, Ward RW. (eds) Proceedings of the 2nd International Symposium on Fusarium head blight. USWBSI, Orlando FL., p. 330.
Leiteritz R, Focke I. 1977. Praktische Hinweise zur Bonitur der Spelzenbräune und der partiellen Weissährigkeit des Weizens. Nachrbl. F. d. Pflschutz der DDR. 31:187-188.
Lemmens M, Krska R, Buerstmayr H, Josephs R, Schuhmacher R, Grausgruber H, Ruckenbauer P. 2003. Fusarium head blight reactions and accumulation of deoxynivalenol, moniliformin and zearalenone in wheat grains. Cereal Research Communications 31:407- 414.
Mankeviciene A, Gaurulcikiene IG, and Suproniene S. 2008. The infestation of winter rye and triticale grain with Fusarium fungi as affected by fungicide use. Cereal Research Communications 36, Suppl. B, 683-687.
Maufras JY, Maumené C, Bourdin MM, Leroux MM. 1994. Fongicides céréales et protéagineux 1994. ITCF, ISBN 2.86492.100.5. 144 pp.
Mauler-Machnik A, Zahn K. 1994. Ear fusarioses in wheat - new findings on their epidemiology and control with Folicur ® (tebuconazole). Pflanzenschutz-Nachrichten Bayer 47:129-155.
McMullen M, Milus G, Prom L. 1999. 1999 Uniform fungicide trials to identify products effective against Fusarium head blight in wheat. In: Wagester JA, Ward RW, Hart LP, Hazen SP, Lewis J, Borden H. (eds) 1999 National Fusarium Head Blight Forum, Sioux Fall, USWBSI, South Dakota, pp. 64-68.
McMullen M, Schatz B, Stover R, Gregoire T. 1997. Studies on fungicide efficacy, application timing and application technologies to reduce Fusarium head blight and deoxynivalenol. Cereal Research Communications 25:779-780.
Mesterházy A. 2010. Chemical control of Fusarium head blight of wheat, a comprehensive view. In Press.
Mesterházy Á. 1984. Fusarium species of wheat in South Hungary, 1970-1983. Cereal Research Communications 12: 167-170.
Mesterházy Á. 1987. Selection of head blight resistant wheat through improved seedling resistance. Plant Breeding 98:25-36.
Mesterházy Á. 1995. Types and components of resistance against Fusarium head blight of wheat. Plant Breeding 114:377-386.
Mesterházy Á. 2002. Role of deoxynivalenol in aggressiveness of Fusarium graminearum and F. culmorum and in resistance to Fusarium head blight. European Journal of Plant Pathology 108:675-684.
Mesterházy Á. 2003. Breeding wheat for Fusarium head blight resistance in Europe. In: Leonard, K. and Bushnell, W. (eds): Fusarium head blight of wheat and barley. APS Press, St. Paul, 211-240
Mesterházy Á. 2003a. Control of Fusarium head blight of wheat by fungicides. In: Leonard, K. and Bushnell, W. (eds) Fusarium head blight of wheat and barley, APS Press, St. Paul. pp. 363-380.
Mesterházy Á. Bartók T. 1996. Control of Fusarium head blight of wheat by fungicides and its effect on the toxin contamination of the grains. Pflanzenschutz-Nachrichten Bayer 49:181-197.
Mesterházy Á, Bartók T, Mirocha CM, Komoróczy R. 1999. Nature of resistance of wheat to Fusarium head blight and deoxynivalenol contamination and their consequences for breeding. Plant Breeding 118: 97-110.
Mesterházy Á, Bartók T. Lamper Cs. 2003. Influence of cultivar resistance, epidemic severity, and Fusarium species on the efficacy of fungicide control of Fusarium head blight in wheat and deoxynivalenol (DON) contamination of grain. Plant Disease 87:1107-1115.
Mesterházy Á, Bartók T, Kászonyi G, Varga M, Tóth B, Varga J. 2005. Common resistance to different Fusarium spp. causing Fusarium head blight in wheat. European Journal of Plant Pathology 112:267-281.
Mesterházy Á, Buerstmayr H, Tóth B, Lehoczki-Krsjak Sz, Szabó-Hevér Á, Lemmens M, 2007. In: Clear R. (ed) Proceedings of the 5th Canadian Workshop on Fusarium Head Blight, Delta Winnipeg, pp. 51-66.
Miedaner T. 1997. Breeding wheat and rye for resistance to Fusarium diseases. Plant Breeding 116:201-220.
Miedaner T, Schneider B, Geiger HH. 2003. Deoxynivalenol (DON) content and Fusarium head blight resistance in segregating populations of winter rye and winter wheat. Crop Science 43:519-526.
Parry DW, Jenkinson P, McLeod L. 1995. Fusarium ear blight (scab) in small grain cereals - a review. Plant Pathology 44:207-238.
Paul PA, Lipps PE, Madden LV. 2005. Relationship between visual estimates of Fusarium head blight intensity and deoxynivalenol accumulation in harvested wheat grain: A metaanalysis. Phytopathology 95:1225-1236.
Polisenska I, Tvaruzek L. 2007. Relationships between deoxynivalenol content, presence of kernels infected by Fusarium spp. pathogens and visually scabby kernels in Czech wheat in 2003-2005. Cereal Research Communications 35:1437-1448.
Ruden BE, Draper MA, Ruden KR. 2005. Sprayer nozzle configurations and effects on fungicide spray deposition on wheat heads. Page 230. (Abstr.). In: Canty, S.M., Boring, T., Wardwell, J., Siler, L. and Ward, R.W. (eds) Proceedings of the 2005 National Fusarium Head Blight Forum, USWBSI, Milwaukee, 245 pp
Scott IT. 1927. Varietal resistance and susceptibility to wheat scab. Univ. Minnesota, Agricultural Experimental Station Research Bulletin No. 111. 14 pp.
Snijders CHA. 1990a. Genetic variation for resistance to Fusarium head blight in bread wheat. Euphytica 50:171-179.
Snijders CHA. 1990b. The inheritance of resistance to head blight caused by Fusarium culmorum in winter wheat. Euphytica 50:11-18.
Snijders CHA. 2004. Resistance in wheat to Fusarium infection and trichothecene formation Toxicology Letters 153: Special Issue, 37-46.
Sommer C, Steiner U, Oerke EC, Dehne HW. 2008. Heterogeneity in the occurrence of Fusarium spp. in wheat. Cereal Research Communications 36: Suppl. B. 643-644.
Stepien L, Popiel D, Koczyk G, Chelkowski J. 2008. Wheat -infecting Fusarium species in Poland - their chemotypes and frequencies revealed by PCR assay. Journal of Applied Genetics 49:433-441.
Sváb J. 1973. Biometriai módszerek a kutatásban (Methods for biometrics in research). Mezigazdasági Kiadó, Budapest.
Tóth B, Kászonyi G, Bartók T, Varga J, Mesterházy Á. 2008. Common resistance of wheat to members of the Fusarium graminearum species complex and F. culmorum. Plant Breeding, 127:1-8.
Weber E. 1967. Grundriss der biologischen Statistik. VEB Fisher Verlag, Jena (GDR).
Wilcoxson RD. 1996. Fungicides for control of Fusarium head blight - a review. Paper No. 22507 of the Minnesota Agr. Exp. Sta, St. Paul. Agr. Exp. Stn. University of Minnesota.
Zhou CF. 1985. Production constrains and research priorities in the southern winter wheat region of China. Pages 72-77. In: Wheat for more tropical Environments, CIMMYT, Mexico D.F.
Table 1. Fungicides and their active ingredients, 2005-2008.
Table 2. Fusarium reaction of several selected genotypes in 2006.
Table 3. Summary of the 2006 variety registration tests, means for four isolates and inoculation dates. R1-R4: control lines from Szeged.
Table 4. Performance of the control cultivars in the variety registration test, 2008
Table 5. Fungicides against FHB in wheat, DON contamination (mgkg-1) across years (2006- 2008) and four isolates
Table 6. Fungicides against FHB in wheat, DON contamination (mgkg-1) across varieties (2006-2008) and years, small plot test with artificial inoculation
Table 7. Fungicides against FHB in wheat, DON contamination (mgkg-1) across varieties (2006-2008) and years. Farm scale test with natural infection.
Figure 1. Relation between FDK and DON data of the 2005 resistance test series, means for four isolates, n=117
Figure 2. Regression between FDK and DON contamination in the 2007 variety registration test.
Figure 3. Regression between FDK and FHB values of variety candidates - non Fusarium program products - in 2008
Figure 4. Fusarium traits for the variety candidates of the 3rd year; means of four isolates, 2008.
Figure 5. DON contamination (mgkg-1) of commercial cultivars 2006-2008, means across years and four isolates
Figure 6. Comparison of DON contamination of the small plot and farm scale trials at different fungicides against Fusarium head blight, 2006-2008






.jpg&w=3840&q=75)