Mycotoxins and their effect on poultry production
Published: January 1, 1900
By: K. Jewers
1. - Introduction
Mycotoxins are a diverse group of toxic secondary metabolites produced by certain moulds when they
grow on agricultural products. They do not belong to a single class of chemical compounds, and they
differ in their toxicological effects. Some mycotoxicoses, the toxic manifestations of mycotoxins in humans or animals, have been known for hundreds of years (e.g.ergotism).However, it wastheoutbreak of Turkey X disease in the United Kingdom during 1960, and the subsequent discovery of the aflatoxins and their toxic effects by scientists at TDRl and other institutions, which prompted the study of mycotoxins as a specialised field of scientific activify (Coker, 1979).
II. - Aflatoxin
It was the death of about 100,000 turkey poults in the United Kingdom in 1960 following the ingestion of a poultry feed containing Brazilian groundnut cake which led to the discovery of a group of compounds now called the aflatoxins.Chemical and microbiological investigations soon revealed that the toxic effects produced by the Brazilian ground nut cake had resulted from the presence of quantities of four secondary metabolites of the mould Aspergillus flaws in the diet. As these compounds fluoresced either green or blue in ultraviolet light,they were designated aflatoxin B1 (la), aflatoxin B2 (1 b), aflatoxin G1, (2a), and aflatoxin G2 (2b).

Almost all agricultural commodities will support the growth of the aflatoxin-producing fungi Aspergillus flavus and A. parasiticus. Formation of aflatoxins can occur during the pre- and post-harvest stages of food production as long as a suitable environment for mould growth is provided. Optimal conditions for aflatoxin production are a water activity in excess of 0.85 and a temperature of 27OC, conditions which are frequently encountered in the Mediterranean region. Different crops vary in their ability to support fungal colonisation because of differences in thechemical composition of each commodity. The incidence and magnitude of aflatoxin contamination varies with seasonal and geographical factors and also with the conditions under which the crop is grown, harvested, stored and transported (Jewers et al., 1986).
1. The toxicity of aflatoxins
a) Acute toxicity
The acute toxicity of aflatoxin BI in poultry varies from species to species. Thus, the LD50 single dose
(mg/kg body weight) is 0.3 for ducklings,and 6.0-16.0 for chickens.The acute toxicity of aflatoxins is
governed by the age, sex and strain of the animal, its condition,.the route of administration, the
composition of the diet, the environment during the test, and the time elapsed before the LD50 is
measured. Table 1 shows the acute toxicities of the four aflatoxins in one day old ducklings.
b) Chronic toxicity
Aflatoxin B1 is probably the most potent naturally occuring hepatic carcinogen known, and it is for this reason that many countries have introduced legislation restricting the amount of this compound allowed in foods and feeds. Table 2 records the level of aflatoxin B1 in the diet which has resulted in liver lesions in chicken, turkey poults and ducklings (Coker, 1979).
A variety of other chronic effects has been reported for aflatoxin B1 when fed to poultry. Edds et al. (1973) have reported that poultry diets containing 250-500 ug/kg ofaflatoxins,when fed to poultry, predisposed them to attack by bacteria and viruses. This is consistent with the known effect of aflatoxin on the immune system as a result of action on theT-cells (Pier et al., 1979). Aflatoxinappears to have adose-related effect on the lymphatic tissues of poultry. Thymic involution of poults, without an associated effect on the bursa component of lymph tissue, has been induced by moderate levels of aflatoxin (0.5 ppm Bl).
Impaired function of lymphocytes and phagocytes appears to be the major aflatoxin-induced deficiency in immunogenesis.
Aflatoxin has been reported to affect the uptake of a number of essential components of the diet, such as coccidiostats,vitamins and aminoacids. This can have a disastrous effecton poultry health and production.
2. The detection of aflatoxin in feedstuffs
The European Community (EC) adopted a directive in 1976 restricting the levels of aflatoxin permitted in animal feeds. For straights, a maximum BI content of 50 ug/kg was imposed, and for whole feed, levels of 20 ug/kg and 10 ug/kg were set for poultry and chicks respectively. In 1986, the regulations were extended to include six commodities used as raw materials in the manufacture of animal feeds : babassu, copra, cotton seed, ground nut, maize and palm kernels. A maximum level of 200 ug/kg of aflatoxin was set for these commodities.
Problems are encountered in the determination of aflatoxin levels in raw materials used in animal feed production due to the highly skewed distribution of aflatoxin in bulk commodities. As a result of the low levels of aflatoxin allowed in raw materials, and the heterogeneous distribution of aflatoxin contaminated particles through out the mass, large samples have to be collected and processed. In order to ensure that representative samples are collected, and that there presentative nature of the samples is maintained during sample division and analysis, sampling, sample preparation and analytical methodologies are required for each commodity. Unfortunately, these are not available at the present time, although we are currently working on the problem, and so the strict enforcement of the EC regulations is difficult (Jewers, 1987).
3. Decontamination of commodities
Various methods have been tried to decontaminate aflatoxin contaminated commodities (e.g. ground nut, cotton seed, palm kerne lcake/meal and maize). These include physical methods (sorting, irradiation techniques, heating), chemical methods (acids, bases, oxidising agents), biological methods (microbiological), and solvent extraction (Coker,1986). Ammonia gas appears to be the most promising approach as it is capable of reducing the aflatoxin level in situ by more than 95% and is applicable to a variety of contaminated commodities using batch and continous processing methods (Coker et al., 1985).
Extensive work has been carried out on the toxicology of ammoniated aflatoxin contaminated commodities, including studies in poultry,but so far the regulatory authorities in Europeand the USA have not been willing to approve the use of these materials in animal feeds. However, in France and certain states in the USA, ammoniated aflatoxin contaminated commodities are being used in selected animal feeds with no reported toxic manifestations.
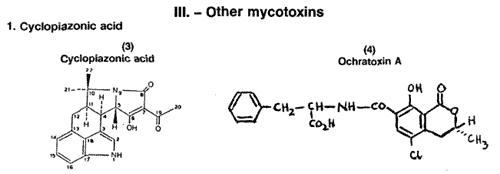
Cyclopiazonicacid (3) was first discovered as aresult of ascientific investigation of the secondary
metabolies of Penicillium cyclopium(P. griseofulvum). Subsequent investigations revealed that it is
produced by several economically important fungi including A. flavus (Luk et al., 1977). Recent
toxicological studies have shown that the principal target organs of this micotoxin are the liver, kidney and digestive system, with liver nvolvement occuring primarily in rats. Feeding trials with chicken shave produced mucosal necrosis and inflammation in the crop, proventriculus and gizzard. In these chickens the kidneys often were swollen or distended.
Cole (1986) has reappraised the etiology of the classical Turkey X diseases syndrome and has suggested that the arching. of the neck and the head drawn back (opisthotonus), and the legs stretched (extended) fully backwards are effects produced by cyclopiazonic acid but not by aflatoxins. Thus he concludes that this mycotoxinco-occured with the aflatoxins in the Brazilian ground nut cake responsible forTurkey X disease.
2. Ochratoxin
The ochratoxins were discovered as a result of mouldy corn cultures being fed to ducklings, rats and mice (Theron et al., 1966), and ochratoxin A (4) was later found to be the major toxic component of Aspergillus oebraccus (Doupnik and Peckham, 1970 ; Peckham et al., 1971). It has since been shown that ochratoxin is produced by numerous species of Aspergillus and Penicillium. In recent years, ochrotoxicosis, the disease state induced by ochratoxin, has been reported in broilers, layers, and turkeys from seven different states in the USA, with levels of ochratoxin in feed ranging from 0.3 to 16 ug/g (Wyatt, 1979).
The minimal dietary growth inhibitory dose for. young broiler chicks is 2 ug/g for ochratoxin. This compares with 2.5 and 4 ug/g for aflatoxin and T-2 toxin respectively. Visible examination of the chicks showed no clinical symptoms due to ochratoxin, but the study of affected broiler chickens has shown severe hydration and emaciation, proventricular haemorrhages, and visceral gout with white urate deposits through out the body cavity and internal organs. The main target organ is the kidney, with the liver also affected to a lesser degree. The kidneys of affected birds become enlarged, and the livers exhibit a tan coloured appearance, have an increased glycogen content, but the liver fat does not increase. Inhibition of liver phosphorylase and decreased mitochondrial respiration are thought to be responsible for the increased liver glycogen.
Aflatoxicosis and ochratoxicosis result in a rubbery condition of the bones apparently related to increased tibial diameters and perhaps poor mineralization of bone tissue in young broiler chicks.
A variety of toxic manifestations has been reported for mature laying hens fed 1 to 4 g/g of ochratoxin.
These include a decrease in egg production, food consumption and serum protein concentrations, and an increase in prothrombin times.
3. Citrinin
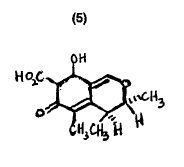
Likeochatroxin, citrinin (5) is an ephrotoxin. It is produced by several species of Penicillium and
Aspergillus. Reduced growth rates, decreased feed and water consumption have been observed as toxic manifestations of this mycotoxin. Decreased feed intake was observed with 62.5 ug/g of citrinin, whereas body weight and water intake were affected by 500 and 250 ug/g respectively in chickens. Two week old chicks have been found to increase their water intake by about two-fold in four hours when fed 500 ug/g of citrinin. Acute diarrhoea follows. Removal of the citrinin' from the diet results in a return to normal water consumption and a subsidence of the diarrhoea in about six hours.
Nephrotoxicity and hepatotoxicity in chickens occurs at dietary levels of 250 ug/g of citrinin with liver and kidney enlargements of 1 1 and 22% respectively. Serum sodium levels are also changed. Necropsy of affected birds revealed the presence of pale and swollen kidneys (Wyatt, 1979).
4. Tricothecenes
The ability of the three tricothecenes, diacetoxyscirpenol, croticin and T-2 toxin, to cause oral necrosis and to affect body weight gain has been studied in growing chicks. At adietary inclusionrate of 5 ug/gof
diacetoxyscirpenol, a body weight reduction of 24% resulted, where as T-2 toxin produced an 11% reduction at the same incorporation rate. A more severe oral response was observed with
diacetoxyscirpenol. No effect on body weight gain or oral inflammation or necrosis was observed with croticin fed at 10 ug/g. It is generally regarded that the presence of oral esions in poultry is the primary means of diagnosing tricothecene toxicoses in the field.
5. Slaframine
Slaframine, l-acetoxy-6-aminohydrondolizine, is a parasympathomimetic secretogogue produced by the mould Rhizoctonia ieguminicoia. A recent study of chronic toxicity in broiler chicks has shown that daily oral intubation in saline for 21 days to 240 chicks at 8.8 or 17.8 ug/g body weight does not affect weight, feed intake and utilization, pancreatic weight, liver weight, and small intestine weight. However, changes in digestive' function were observed : the protein content of the digesta, the digest alipase and specific activities, and the digest atrypsin-specific activities were lowered at the higher dose level (Froetschel, 1987).
IV. - The effect of mycotoxins on poultry production
The most obvious effect of mycotoxins on poultry production is mortality, as this can be readily diagnosed and quantified. The outbreak of Turkey X disease resulted in the death of 100,000 turkey poults and many ducks, chickens and pheasants. The total annihilation of 15,000 turkeys in North Carolina by aflatoxin, and the death of 59% of a 16,000-flock of turkeys from ochratoxicosis have also been reported.
However, it is the chronic effects of mycotoxins which could pose the most problems to poultry producers.
Feed refusal which is a rapid, direct response to the presence of mycotoxin in the feed, can be a problem with some animals. In poultry, aflatoxin has been found to be associated with the "bruising" syndrome of broiler chickens. The development of bruises and haemorrhages on the process inglines can result in a downgrading and condemnation of birds and customer dissatisfaction due to infernal bruising not visible to the inspectors. Aflatoxin reduces the fórce required to produce a bruise due to increased capillary fragility
and reduced ability of supporting tissue to cushion the blood vessels against blows. It is important to remember that aflatoxin causes a predisposition to bruising and not the manifestation itself, which is elicited by handling procedures that are innocuous to normal birds.
Growth inhibition is another important toxic effect of mycotoxins in poultry feed; A diet high in protein largely prevents the inhibitory growth caused by aflatoxin, but at a cost.
The concentration of bile required for lipid absorption and digestion from the intestinal tract is decreased during aflatoxicosis. The practical consequence of the malabsorption is to decrease the efficiency of feed conversion into animal products and hence increase the cost of production.
Mycotoxin and vitamins interaction in animal nutrition is unpredictable. Thus, aflatoxin reduces the vitamin A content of chicken livers and serum, but does not appear to enhance or provoke vitamin A deficiency.
Supplementing the diet with vitamin A does not improve the growth rate. During aflatoxicosis, the plasma calcium concentration is lowered byupto 20%, and a dietdeficient in vitamin D3 makes chickens sensitive to doses of aflatoxin too small to retard growth in an adequate diet. Both aflatoxin and ochratoxin decrease the force required to break bones, and this confirms field cases of rickets attributed to the presence of mycotoxins in poultry diets. Vitamins E and K involvement in mycotoxicosis is uncertain, and deficiencies of riboflavin and thiamin interact contradictorily with aflatoxicosis.
Impairment of the mechanisms of resistance to infectious agents is oneof the more insidious effects of mycotoxins. Aflatoxin, ochratoxinand T-2 have all been shown to act upon the immune system, and so their presence in poultry diets could have disasterous consequences.
Egg production can also be affected by mycotoxins in the diet. Aflatoxin apparently inhibits egg production at the point of commitment of an ovum to maturation ; ova already committed are not influenced apart from less yoke than normal being deposited. Decreased egg production, with thin, rubbery shells which break more readily than normal have been observed during field out breaks ofochratoxin. T-2toxicosis in a population of 120,000 hens produced a decrease in production rate from 72 to 51 YO, an increase in egg breakage from the normal 3% to 15% and a further 18% broken in transit to the customer, blood spots increased from essentially zero to 3'30, and 85% of the birds were found to have oral lesions. The result was lost contracts, litigation involving a $1 million plus law suit, slaughter of the flocks, and forced closure of the business.
Aflatoxin has also been implicated in hatchability,impaired semen quality in White Leghorn strains,under pigmentation, altering the response of chickens to environmental extremes and increased sensitivity to sodium chloride. In addition to affecting pigmentation, ochratoxin decreases the tensile strength of the large intestine by decreasing its collagen content and thus causes the fragile large intestine to rupture during processing (Hamilton, 1982).
V. - Conclusion
It is clear that the presence of mycotoxins in poultry feed can result in significant economic losses for the poultry producer. In order to prevent such losses, the EC has introduced regulations limiting the amount of aflatoxin in poultry feeds, and has set maximum levels for aflatoxin in six major raw material sused in animalfeed production: babassu, copra, cotton seed, groundnut, maize and palm kernels. However, the sampling, sample preparation and analytical methodologies required for the monitoring of aflatoxin in these commodities are not available at present, and further work is required to ensure that the heterogeneous distribution of aflatoxin in a commodity is taken into account when newquality control procedures are developed.
At the present time no limits have been set for the other mycotoxins known to produce adverse effects in poultry. Care must be taken by animal feed processors and poultry producers to ensure mycotoxins do not enter the food chain, and this may necessitate them introducing quality control procedures even though a legal framework for such testing is not in place.
Utilization of highly contaminated raw materials presents a major problem. Blending of highly contaminated and uncontaminated raw materials is not advisable, as most mixing techniques involving unground material are likely to lead to pockets of highly contaminated material which could have a disasterous effect on poultry production. An alternative strategy would be to decontaminate the highly contaminated raw material or the feed prior to the addition of vitamins and other additives. We are currently investigating this approach in a project in Pakistan.
References
Coker, R.D., 1979.- Aflatoxin : past, present and future.- Trop. Sci., 21, pp. 143-162.
Coker, R.D. ; Jewers,K. ; Jones,B.D.,1985.- Thedestructionofaflatoxin by ammonia : practical possibilities.- Trop. Sci., 25, pp. 139-154.
Coker, R.D. ; Jewers,K. ; Jones,B.D.,1986.- The treatment ofaflatoxincontaminatedcommodities.- Internat. Biodeterioration, 22S, pp. 103-1 08.
Cole, R.J., 1986.- Etiology of Turkey X disease in retrospect : a case for the involvement of CYClOpiazOniC acid.- Mycotoxin Res., 2, pp. 3-7.
Doupnik, B. ; Peckham,J.C.,1970.-Mycotoxicityof Aspergillus ochraceus to chikcs.- Appl. Microbiol., 19, pp. 594-597.
Edds,G.T. ; Nair,K.P.C. ; Simpson,C.F.,1973.- Effects of aflaaflatoxin B,on resistance inpoultry against cecal coccidiosis and Marek’s disease.- Amer. J. Vet. Res., 34, pp. 819-826.
Froetschel, M.A. ; Hagler,Jr.,W.M. ; Croom,Jr.,W.J. ; Ort, J. ; Broquist,H.P.,1987.- Effects of chronic administration of slaframine on production and digestive function in broiler chicks.- Poultry Sci., 66, pp. 357-362.
Hamilton, P.B., 1982.- Mycotoxins and farm animals.- Refuah. Vet., 39, pp. 17-45.
Jewers,K. ; Coker,R.D. ; Blunden, G. ; Jazwinski,Mary J. ; Sharkey,Angela J., 1986.- Problems involved in the determination of aflatoxin levels in bulk commodities.- lnternat. Biodeteriorafion, 22S, pp. 83-88.
Jewers, K.,1987.- Problems in elation to sampling of consignments for mycotoxin determination and interpretation of results. Proc. 2nd International Conference on Mycotoxins, 28 September - 2nd October, Bangkok, Thailand, F.A.O.,Rome.
Luk, K.C. ; Kobbe,B. ; Townsend,J.M., 1977.- Production of cyclomiazonic acid by Aspergillus flavus Link.- Appl. fnvirn. Microbiol., 33, pp. 21 1-212.
Peckham,J.C. ; Doupnik,B. ; Jones,Jr.,O.H., 1971 .- Acute toxicity of ochratoxins A and Binchicks.- Appl: MiCrObiOl., 21, pp. 492-494.
Mycotoxins are a diverse group of toxic secondary metabolites produced by certain moulds when they
grow on agricultural products. They do not belong to a single class of chemical compounds, and they
differ in their toxicological effects. Some mycotoxicoses, the toxic manifestations of mycotoxins in humans or animals, have been known for hundreds of years (e.g.ergotism).However, it wastheoutbreak of Turkey X disease in the United Kingdom during 1960, and the subsequent discovery of the aflatoxins and their toxic effects by scientists at TDRl and other institutions, which prompted the study of mycotoxins as a specialised field of scientific activify (Coker, 1979).
II. - Aflatoxin
It was the death of about 100,000 turkey poults in the United Kingdom in 1960 following the ingestion of a poultry feed containing Brazilian groundnut cake which led to the discovery of a group of compounds now called the aflatoxins.Chemical and microbiological investigations soon revealed that the toxic effects produced by the Brazilian ground nut cake had resulted from the presence of quantities of four secondary metabolites of the mould Aspergillus flaws in the diet. As these compounds fluoresced either green or blue in ultraviolet light,they were designated aflatoxin B1 (la), aflatoxin B2 (1 b), aflatoxin G1, (2a), and aflatoxin G2 (2b).

Almost all agricultural commodities will support the growth of the aflatoxin-producing fungi Aspergillus flavus and A. parasiticus. Formation of aflatoxins can occur during the pre- and post-harvest stages of food production as long as a suitable environment for mould growth is provided. Optimal conditions for aflatoxin production are a water activity in excess of 0.85 and a temperature of 27OC, conditions which are frequently encountered in the Mediterranean region. Different crops vary in their ability to support fungal colonisation because of differences in thechemical composition of each commodity. The incidence and magnitude of aflatoxin contamination varies with seasonal and geographical factors and also with the conditions under which the crop is grown, harvested, stored and transported (Jewers et al., 1986).
1. The toxicity of aflatoxins
a) Acute toxicity
The acute toxicity of aflatoxin BI in poultry varies from species to species. Thus, the LD50 single dose
(mg/kg body weight) is 0.3 for ducklings,and 6.0-16.0 for chickens.The acute toxicity of aflatoxins is
governed by the age, sex and strain of the animal, its condition,.the route of administration, the
composition of the diet, the environment during the test, and the time elapsed before the LD50 is
measured. Table 1 shows the acute toxicities of the four aflatoxins in one day old ducklings.
b) Chronic toxicity
Aflatoxin B1 is probably the most potent naturally occuring hepatic carcinogen known, and it is for this reason that many countries have introduced legislation restricting the amount of this compound allowed in foods and feeds. Table 2 records the level of aflatoxin B1 in the diet which has resulted in liver lesions in chicken, turkey poults and ducklings (Coker, 1979).
A variety of other chronic effects has been reported for aflatoxin B1 when fed to poultry. Edds et al. (1973) have reported that poultry diets containing 250-500 ug/kg ofaflatoxins,when fed to poultry, predisposed them to attack by bacteria and viruses. This is consistent with the known effect of aflatoxin on the immune system as a result of action on theT-cells (Pier et al., 1979). Aflatoxinappears to have adose-related effect on the lymphatic tissues of poultry. Thymic involution of poults, without an associated effect on the bursa component of lymph tissue, has been induced by moderate levels of aflatoxin (0.5 ppm Bl).
Impaired function of lymphocytes and phagocytes appears to be the major aflatoxin-induced deficiency in immunogenesis.
Aflatoxin has been reported to affect the uptake of a number of essential components of the diet, such as coccidiostats,vitamins and aminoacids. This can have a disastrous effecton poultry health and production.
2. The detection of aflatoxin in feedstuffs
The European Community (EC) adopted a directive in 1976 restricting the levels of aflatoxin permitted in animal feeds. For straights, a maximum BI content of 50 ug/kg was imposed, and for whole feed, levels of 20 ug/kg and 10 ug/kg were set for poultry and chicks respectively. In 1986, the regulations were extended to include six commodities used as raw materials in the manufacture of animal feeds : babassu, copra, cotton seed, ground nut, maize and palm kernels. A maximum level of 200 ug/kg of aflatoxin was set for these commodities.
Problems are encountered in the determination of aflatoxin levels in raw materials used in animal feed production due to the highly skewed distribution of aflatoxin in bulk commodities. As a result of the low levels of aflatoxin allowed in raw materials, and the heterogeneous distribution of aflatoxin contaminated particles through out the mass, large samples have to be collected and processed. In order to ensure that representative samples are collected, and that there presentative nature of the samples is maintained during sample division and analysis, sampling, sample preparation and analytical methodologies are required for each commodity. Unfortunately, these are not available at the present time, although we are currently working on the problem, and so the strict enforcement of the EC regulations is difficult (Jewers, 1987).
3. Decontamination of commodities
Various methods have been tried to decontaminate aflatoxin contaminated commodities (e.g. ground nut, cotton seed, palm kerne lcake/meal and maize). These include physical methods (sorting, irradiation techniques, heating), chemical methods (acids, bases, oxidising agents), biological methods (microbiological), and solvent extraction (Coker,1986). Ammonia gas appears to be the most promising approach as it is capable of reducing the aflatoxin level in situ by more than 95% and is applicable to a variety of contaminated commodities using batch and continous processing methods (Coker et al., 1985).
Extensive work has been carried out on the toxicology of ammoniated aflatoxin contaminated commodities, including studies in poultry,but so far the regulatory authorities in Europeand the USA have not been willing to approve the use of these materials in animal feeds. However, in France and certain states in the USA, ammoniated aflatoxin contaminated commodities are being used in selected animal feeds with no reported toxic manifestations.

Cyclopiazonicacid (3) was first discovered as aresult of ascientific investigation of the secondary
metabolies of Penicillium cyclopium(P. griseofulvum). Subsequent investigations revealed that it is
produced by several economically important fungi including A. flavus (Luk et al., 1977). Recent
toxicological studies have shown that the principal target organs of this micotoxin are the liver, kidney and digestive system, with liver nvolvement occuring primarily in rats. Feeding trials with chicken shave produced mucosal necrosis and inflammation in the crop, proventriculus and gizzard. In these chickens the kidneys often were swollen or distended.
Cole (1986) has reappraised the etiology of the classical Turkey X diseases syndrome and has suggested that the arching. of the neck and the head drawn back (opisthotonus), and the legs stretched (extended) fully backwards are effects produced by cyclopiazonic acid but not by aflatoxins. Thus he concludes that this mycotoxinco-occured with the aflatoxins in the Brazilian ground nut cake responsible forTurkey X disease.
2. Ochratoxin
The ochratoxins were discovered as a result of mouldy corn cultures being fed to ducklings, rats and mice (Theron et al., 1966), and ochratoxin A (4) was later found to be the major toxic component of Aspergillus oebraccus (Doupnik and Peckham, 1970 ; Peckham et al., 1971). It has since been shown that ochratoxin is produced by numerous species of Aspergillus and Penicillium. In recent years, ochrotoxicosis, the disease state induced by ochratoxin, has been reported in broilers, layers, and turkeys from seven different states in the USA, with levels of ochratoxin in feed ranging from 0.3 to 16 ug/g (Wyatt, 1979).
The minimal dietary growth inhibitory dose for. young broiler chicks is 2 ug/g for ochratoxin. This compares with 2.5 and 4 ug/g for aflatoxin and T-2 toxin respectively. Visible examination of the chicks showed no clinical symptoms due to ochratoxin, but the study of affected broiler chickens has shown severe hydration and emaciation, proventricular haemorrhages, and visceral gout with white urate deposits through out the body cavity and internal organs. The main target organ is the kidney, with the liver also affected to a lesser degree. The kidneys of affected birds become enlarged, and the livers exhibit a tan coloured appearance, have an increased glycogen content, but the liver fat does not increase. Inhibition of liver phosphorylase and decreased mitochondrial respiration are thought to be responsible for the increased liver glycogen.
Aflatoxicosis and ochratoxicosis result in a rubbery condition of the bones apparently related to increased tibial diameters and perhaps poor mineralization of bone tissue in young broiler chicks.
A variety of toxic manifestations has been reported for mature laying hens fed 1 to 4 g/g of ochratoxin.
These include a decrease in egg production, food consumption and serum protein concentrations, and an increase in prothrombin times.
3. Citrinin

Likeochatroxin, citrinin (5) is an ephrotoxin. It is produced by several species of Penicillium and
Aspergillus. Reduced growth rates, decreased feed and water consumption have been observed as toxic manifestations of this mycotoxin. Decreased feed intake was observed with 62.5 ug/g of citrinin, whereas body weight and water intake were affected by 500 and 250 ug/g respectively in chickens. Two week old chicks have been found to increase their water intake by about two-fold in four hours when fed 500 ug/g of citrinin. Acute diarrhoea follows. Removal of the citrinin' from the diet results in a return to normal water consumption and a subsidence of the diarrhoea in about six hours.
Nephrotoxicity and hepatotoxicity in chickens occurs at dietary levels of 250 ug/g of citrinin with liver and kidney enlargements of 1 1 and 22% respectively. Serum sodium levels are also changed. Necropsy of affected birds revealed the presence of pale and swollen kidneys (Wyatt, 1979).
4. Tricothecenes

The ability of the three tricothecenes, diacetoxyscirpenol, croticin and T-2 toxin, to cause oral necrosis and to affect body weight gain has been studied in growing chicks. At adietary inclusionrate of 5 ug/gof
diacetoxyscirpenol, a body weight reduction of 24% resulted, where as T-2 toxin produced an 11% reduction at the same incorporation rate. A more severe oral response was observed with
diacetoxyscirpenol. No effect on body weight gain or oral inflammation or necrosis was observed with croticin fed at 10 ug/g. It is generally regarded that the presence of oral esions in poultry is the primary means of diagnosing tricothecene toxicoses in the field.
5. Slaframine
Slaframine, l-acetoxy-6-aminohydrondolizine, is a parasympathomimetic secretogogue produced by the mould Rhizoctonia ieguminicoia. A recent study of chronic toxicity in broiler chicks has shown that daily oral intubation in saline for 21 days to 240 chicks at 8.8 or 17.8 ug/g body weight does not affect weight, feed intake and utilization, pancreatic weight, liver weight, and small intestine weight. However, changes in digestive' function were observed : the protein content of the digesta, the digest alipase and specific activities, and the digest atrypsin-specific activities were lowered at the higher dose level (Froetschel, 1987).
IV. - The effect of mycotoxins on poultry production
The most obvious effect of mycotoxins on poultry production is mortality, as this can be readily diagnosed and quantified. The outbreak of Turkey X disease resulted in the death of 100,000 turkey poults and many ducks, chickens and pheasants. The total annihilation of 15,000 turkeys in North Carolina by aflatoxin, and the death of 59% of a 16,000-flock of turkeys from ochratoxicosis have also been reported.
However, it is the chronic effects of mycotoxins which could pose the most problems to poultry producers.
Feed refusal which is a rapid, direct response to the presence of mycotoxin in the feed, can be a problem with some animals. In poultry, aflatoxin has been found to be associated with the "bruising" syndrome of broiler chickens. The development of bruises and haemorrhages on the process inglines can result in a downgrading and condemnation of birds and customer dissatisfaction due to infernal bruising not visible to the inspectors. Aflatoxin reduces the fórce required to produce a bruise due to increased capillary fragility
and reduced ability of supporting tissue to cushion the blood vessels against blows. It is important to remember that aflatoxin causes a predisposition to bruising and not the manifestation itself, which is elicited by handling procedures that are innocuous to normal birds.
Growth inhibition is another important toxic effect of mycotoxins in poultry feed; A diet high in protein largely prevents the inhibitory growth caused by aflatoxin, but at a cost.
The concentration of bile required for lipid absorption and digestion from the intestinal tract is decreased during aflatoxicosis. The practical consequence of the malabsorption is to decrease the efficiency of feed conversion into animal products and hence increase the cost of production.
Mycotoxin and vitamins interaction in animal nutrition is unpredictable. Thus, aflatoxin reduces the vitamin A content of chicken livers and serum, but does not appear to enhance or provoke vitamin A deficiency.
Supplementing the diet with vitamin A does not improve the growth rate. During aflatoxicosis, the plasma calcium concentration is lowered byupto 20%, and a dietdeficient in vitamin D3 makes chickens sensitive to doses of aflatoxin too small to retard growth in an adequate diet. Both aflatoxin and ochratoxin decrease the force required to break bones, and this confirms field cases of rickets attributed to the presence of mycotoxins in poultry diets. Vitamins E and K involvement in mycotoxicosis is uncertain, and deficiencies of riboflavin and thiamin interact contradictorily with aflatoxicosis.
Impairment of the mechanisms of resistance to infectious agents is oneof the more insidious effects of mycotoxins. Aflatoxin, ochratoxinand T-2 have all been shown to act upon the immune system, and so their presence in poultry diets could have disasterous consequences.
Egg production can also be affected by mycotoxins in the diet. Aflatoxin apparently inhibits egg production at the point of commitment of an ovum to maturation ; ova already committed are not influenced apart from less yoke than normal being deposited. Decreased egg production, with thin, rubbery shells which break more readily than normal have been observed during field out breaks ofochratoxin. T-2toxicosis in a population of 120,000 hens produced a decrease in production rate from 72 to 51 YO, an increase in egg breakage from the normal 3% to 15% and a further 18% broken in transit to the customer, blood spots increased from essentially zero to 3'30, and 85% of the birds were found to have oral lesions. The result was lost contracts, litigation involving a $1 million plus law suit, slaughter of the flocks, and forced closure of the business.
Aflatoxin has also been implicated in hatchability,impaired semen quality in White Leghorn strains,under pigmentation, altering the response of chickens to environmental extremes and increased sensitivity to sodium chloride. In addition to affecting pigmentation, ochratoxin decreases the tensile strength of the large intestine by decreasing its collagen content and thus causes the fragile large intestine to rupture during processing (Hamilton, 1982).
V. - Conclusion
It is clear that the presence of mycotoxins in poultry feed can result in significant economic losses for the poultry producer. In order to prevent such losses, the EC has introduced regulations limiting the amount of aflatoxin in poultry feeds, and has set maximum levels for aflatoxin in six major raw material sused in animalfeed production: babassu, copra, cotton seed, groundnut, maize and palm kernels. However, the sampling, sample preparation and analytical methodologies required for the monitoring of aflatoxin in these commodities are not available at present, and further work is required to ensure that the heterogeneous distribution of aflatoxin in a commodity is taken into account when newquality control procedures are developed.
At the present time no limits have been set for the other mycotoxins known to produce adverse effects in poultry. Care must be taken by animal feed processors and poultry producers to ensure mycotoxins do not enter the food chain, and this may necessitate them introducing quality control procedures even though a legal framework for such testing is not in place.
Utilization of highly contaminated raw materials presents a major problem. Blending of highly contaminated and uncontaminated raw materials is not advisable, as most mixing techniques involving unground material are likely to lead to pockets of highly contaminated material which could have a disasterous effect on poultry production. An alternative strategy would be to decontaminate the highly contaminated raw material or the feed prior to the addition of vitamins and other additives. We are currently investigating this approach in a project in Pakistan.
References
Coker, R.D., 1979.- Aflatoxin : past, present and future.- Trop. Sci., 21, pp. 143-162.
Coker, R.D. ; Jewers,K. ; Jones,B.D.,1985.- Thedestructionofaflatoxin by ammonia : practical possibilities.- Trop. Sci., 25, pp. 139-154.
Coker, R.D. ; Jewers,K. ; Jones,B.D.,1986.- The treatment ofaflatoxincontaminatedcommodities.- Internat. Biodeterioration, 22S, pp. 103-1 08.
Cole, R.J., 1986.- Etiology of Turkey X disease in retrospect : a case for the involvement of CYClOpiazOniC acid.- Mycotoxin Res., 2, pp. 3-7.
Doupnik, B. ; Peckham,J.C.,1970.-Mycotoxicityof Aspergillus ochraceus to chikcs.- Appl. Microbiol., 19, pp. 594-597.
Edds,G.T. ; Nair,K.P.C. ; Simpson,C.F.,1973.- Effects of aflaaflatoxin B,on resistance inpoultry against cecal coccidiosis and Marek’s disease.- Amer. J. Vet. Res., 34, pp. 819-826.
Froetschel, M.A. ; Hagler,Jr.,W.M. ; Croom,Jr.,W.J. ; Ort, J. ; Broquist,H.P.,1987.- Effects of chronic administration of slaframine on production and digestive function in broiler chicks.- Poultry Sci., 66, pp. 357-362.
Hamilton, P.B., 1982.- Mycotoxins and farm animals.- Refuah. Vet., 39, pp. 17-45.
Jewers,K. ; Coker,R.D. ; Blunden, G. ; Jazwinski,Mary J. ; Sharkey,Angela J., 1986.- Problems involved in the determination of aflatoxin levels in bulk commodities.- lnternat. Biodeteriorafion, 22S, pp. 83-88.
Jewers, K.,1987.- Problems in elation to sampling of consignments for mycotoxin determination and interpretation of results. Proc. 2nd International Conference on Mycotoxins, 28 September - 2nd October, Bangkok, Thailand, F.A.O.,Rome.
Luk, K.C. ; Kobbe,B. ; Townsend,J.M., 1977.- Production of cyclomiazonic acid by Aspergillus flavus Link.- Appl. fnvirn. Microbiol., 33, pp. 21 1-212.
Peckham,J.C. ; Doupnik,B. ; Jones,Jr.,O.H., 1971 .- Acute toxicity of ochratoxins A and Binchicks.- Appl: MiCrObiOl., 21, pp. 492-494.
Pier, A.C. ; Richard,J.L. ; Thurston,J .R.,1979.- The influence of mycotoxins on resistance an dimmunity.- Washington DC: Interactions of mycotoxins in animal production, Nat. Acd. Sci., pp. 56-66.
Theron, J.J., vanderMercue,K.J. ; Liebenberg,N. ; Joubert,H.J.B. ; Nel,W.,1966.- Acute liver injury in ducklings and rats as a result of ochratoxin poisoning.- J. Pathol. Bacteriol., 91, pp. 521-529.
Wyatt, R.D., 1979.- Biological effects of mycotoxins (other than aflatoxin) on poultry.- washington DC: lnteraction of mycotoxins in animal production, Nat. Acad. Sci., pp. 87-95.
Wyatt,R.D.,1983.- A review and current knowledge of moulds and mycotoxins in animal feeding stuffs. Peler Hand Seminar, Grims Dyke Hotel, Stanmore, England.
Theron, J.J., vanderMercue,K.J. ; Liebenberg,N. ; Joubert,H.J.B. ; Nel,W.,1966.- Acute liver injury in ducklings and rats as a result of ochratoxin poisoning.- J. Pathol. Bacteriol., 91, pp. 521-529.
Wyatt, R.D., 1979.- Biological effects of mycotoxins (other than aflatoxin) on poultry.- washington DC: lnteraction of mycotoxins in animal production, Nat. Acad. Sci., pp. 87-95.
Wyatt,R.D.,1983.- A review and current knowledge of moulds and mycotoxins in animal feeding stuffs. Peler Hand Seminar, Grims Dyke Hotel, Stanmore, England.
Table 1: Acute toxicities of aflatoxins in one day old ducklings
Aflatoxin | B1 | B2 | G1 | G2 |
I LD50 (mg/kgbodyweight) | 0.36 | 0.78 | 1.70 | 2.45 |
Table 2 Level of aflatoxin B1 producing liver lesions in poultry
Species | Chicken | Duckling | Turkeypoults |
B1 in diet (ug/kg) | 500 | 30 | 300 |
K. JEWERS - Tropical Development and Research Institute (TDRI), London (UK)
Related topics
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post26 de abril de 2008
Thanx for the information about mycotoxins in poultry feed. Today we are facing polyneuropathy syndrome in broiler of 3-4 week age postmortem mycobiota revealed Aspergilluss, ana Penicicillium spp fungus
Dr.Kedar Karki
Central Veterinary Laboratory Katmandu Nepal
Neospark Drugs and Chemicals Pvt.Ltd
9 de enero de 2008
May I know the Maximum permisible, tolerable level, lethal level of various mycotoxins in poultry feed??










.jpg&w=3840&q=75)