Mycotoxins in silages
Published: December 17, 2008
By: Inês Rodrigues (Product Manager Mycofix® line) and Yunior Acosta Aragón (Product Manager for Biomin® BioStabil line) – Courtesy of BIOMIN GmbH
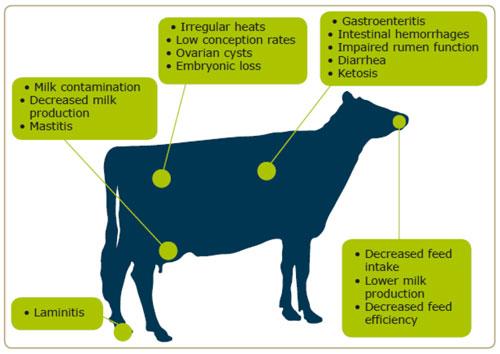
You probably recognise the scheme below. We'd ask you to take another good look at it. Are these problems often present in your herd? Do your cows seldom respond to veterinary therapies? Are the symptoms often associated with specific feedstuffs? If this is the case you are probably facing a mycotoxin problem. As supported by scientific literature, even the best management of agricultural strategies cannot totally eradicate mycotoxin contamination. Mycotoxins are secondary metabolites produced by filamentous fungi which have a negative impact on animals and humans, either by ingestion, inhalation or skin contact. BIOMIN, a company with a long standing tradition (more than 20 years) of research in mycotoxin risk management recently published a newsletter concerning the effects of mycotoxins and their impact on dairy cows (Newsletter 64 "Mycotoxins in dairy cows"). This newsletter was followed up with an overview of the management of silage (see also Newsletter 59, Biomin® BioStabil: a silage additive product line which fits the needs of the producers"), a feedstuff highly prone to mycotoxin contamination due to bad agricultural practice. Let´s try to understand this interesting and multifactorial problem together!
Mycotoxins in silages: occurrence and prevention
Mycotoxins have been isolated from silages that did not show visible mould contamination (Schneweis 2000, Wilkinson 2005). Therefore, in practical terms, it cannot be guaranteed that visually sound silage does not contain mycotoxins as these substances are odourless and invisible. If silage shows spoilage symptoms, it should not be fed to animals to prevent the occurrence of mycotoxicoses, as there is a high probability of mycotoxin contamination in these cases. Instead, it is strongly recommended to discard spoiled silage (30 - 40 cm of the surface).
Mycotoxins in silages: occurrence and prevention
Mycotoxins have been isolated from silages that did not show visible mould contamination (Schneweis 2000, Wilkinson 2005). Therefore, in practical terms, it cannot be guaranteed that visually sound silage does not contain mycotoxins as these substances are odourless and invisible. If silage shows spoilage symptoms, it should not be fed to animals to prevent the occurrence of mycotoxicoses, as there is a high probability of mycotoxin contamination in these cases. Instead, it is strongly recommended to discard spoiled silage (30 - 40 cm of the surface).

The menace of mycotoxins in the silage
As can be seen in Table 1, laboratory analyses of silages confirm the presence of mycotoxins in silage samples. Analyses were performed using standard procedures. Aflatoxins, ZON, DON and total FUM were analyzed by HPLC (High Pressure Liquid Chromatography). For the purpose of data analysis, non-detect levels were based on the quantification limits of the test method for each toxin: Aflatoxin B1 <0.5 μg/kg; ZON <10 μg/kg; DON <150 μg/kg; and FUM <25 μg/kg.
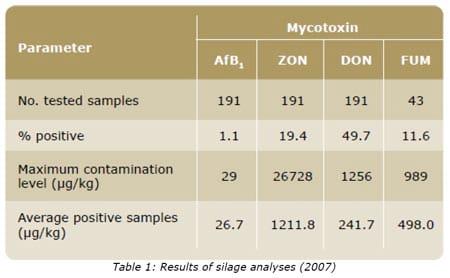
The occurrence of AfB1 and FUM was less frequent than that of others such as ZON and DON. Only 2 samples out of 191 (1.1%) were found positive for AfB1. Scudamore and Livesey (1998) consider that surveillance for aflatoxin in silage and forages has rarely been reported, despite the acknowledged hazardous effects this mycotoxin implies. Aflatoxin degradation in the rumen is generally weak, less than 10% with dosages from 1 to 10 μg/ ml (Yiannikouris and Jouany, 2002). In lactating animals, AfM1 and other metabolites are excreted in the milk (Gratz and Täubel, 2007). This carcinogenic mycotoxin was proven to be related to increased lameness (subclinical mastitis) and impaired fertility (cystic ovaries) (Özsoy et al., 2004). Out of the 43 samples tested for fumonisins, 11.6% presented a positive result. The maximum contamination level found was 989 ppb. FUM has shown to reduce milk production in dairy cattle (Diaz et al. 2000). ZON and/ or DON contamination was found in many samples tested for these mycotoxins. From the 191 samples tested approximately 20% and 40% were positive for ZON and DON, respectively. Levels as high as 26 728 μg/kg (ZON) and 1256 μg/ kg (DON) were found for these mycotoxins. Several case reports have related ZON to an estrogenic response in ruminants and included abortions as a symptom.
Minimizing mycotoxin contamination in the field
Ensiling has become an important process in the conservation of harvested crops. This process is based on anaerobic storage in order to promote the growth of desirable microorganisms (lactic acid bacteria which lead to a deep acidification) and to prevent contamination with undesirable microorganisms (especially Clostridium spp. and Listeria spp. bacteria, moulds and yeasts; Kalac and Woodford, 1982). According to Richter and Bauer (1997), the most frequently occurring mould in corn silage is Penicillium roqueforti (Figure 1), whereas in grass silages Monascus ruber (Figure 2) and Aspergillus fumigatus (Figure 3) are the most common ones. The last two moulds were classified by Pelhate (1977) as tolerant in their tolerance to oxygen, whereas Penicillium roqueforti is considered as microphilic or indifferent to oxygen presence.

Since more than 90 % of the mycotoxins in feed are already produced on the field, the first step to avoid mycotoxins in silages should be done at the site of crop production. Several environmental factors play a role in the growth of moulds on the field: temperature, composition of the gas atmosphere, substrate properties including moisture content and water activity (aw), pH and chemical composition, as well as biotic factors (insects, rodents, and other microorganisms) (Ramakrishna et al. 1993, Ominski et al. 1994).
The use of resistant plants against Fusarium spp. is recommended, as well as a good crop rotation. Rain and high thermal amplitude are supporting risk factors, therefore the weather forecast or the weather conditions should be known as they are valuable sources of information concerning risk management.
The level of field mycotoxins is known to increase with plant maturation. As can be assessed in Figure 4, the contamination level was negligible, from a practical point of view, for the grains with 63.3% of dry matter (DM).
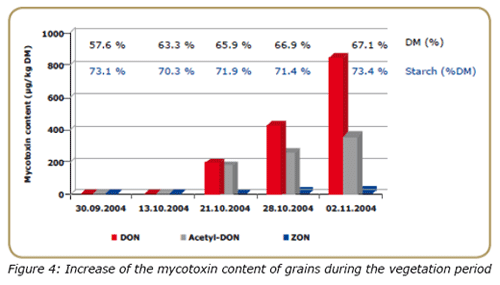
At this maturity stage, 90 % of the starch yield potential is already reached. However, 3 weeks later, the mycotoxin load increased substantially, reaching over 800 μg/kg, 400 μg/kg and approximately 50 μg/kg DM of DON, Acetyl- DON and ZON respectively. This practical example is in accordance with other authors (Jones et al., 1981; Warfield and Gilchrist, 1999), supporting the need for adequate planning of harvesting activities.
Avoiding mycotoxin contamination in the ensiling process
While fusariotoxins are mainly produced on the field, Aspergillus and Penicillium fungi will most likely develop after harvest leading to the production of aflatoxins and ochratoxins, especially under poor storage conditions. However, as most of the toxic compounds present on agricultural commodities will remain stable after harvest under aerobic conditions (Scudamore and Livesey, 1998), crop management should not be discarded as an important factor.
Hygiene (clean crop, clean silo) should be maximized; dirt can considerably increase the number of undesirable microorganisms, namely Clostridia and Listeria, and fungi due to the ubiquitous existence of Fusarium spores in the soil (Schrödter, 2004).
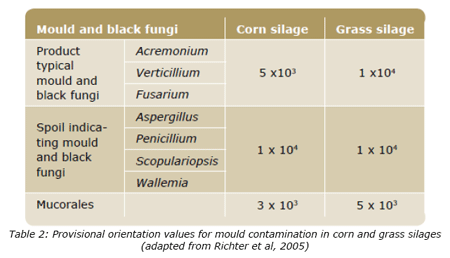
According to Scudamore and Livesey (1998), field-derived fungi will, in time, be replaced by storage fungi, particularly with inadequate drying or if the moisture content is not maintained below about 15%. In the case of silages, the moisture content is 3 to 5 times higher than this value and therefore water activity (aw) is much higher than needed by fungi (0.65 according to the same authors), which will increase the contamination risk. The most common silage materials are grass, corn, whole crop cereals and different industrial byproducts. Authors like Richter et al. (2005) gave provisional orientation values for contaminations with moulds in corn and grass silages (Table 2).
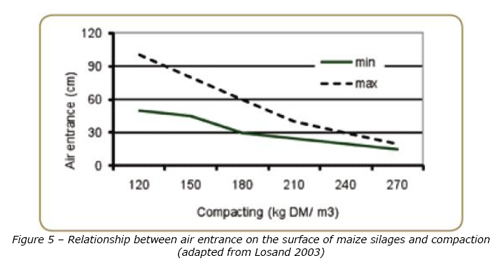
Most of these fungi are known producers of mycotoxins which will persist throughout the ensiling process. In spite of the fact that some moulds can grow even under anaerobic conditions/low amount of oxygen, the creation of anaerobic conditions in the silage can considerably reduce the growth of fungi and subsequently mycotoxin formation. Two aspects are essential to control the oxygen entrance into the silage: compaction and coverage. Compaction eliminates the oxygen inside the material and coverage maintains the silage anaerobically preserved. When the silage is well compacted, the oxygen entrance and penetration will be limited to the layer in contact with the air in the feed out phase (Losand, 2003) (Figure 5) and the aerobic stability will be improved (Kleinmans, 1996).
Crop particle length is closely related to compaction. The "rule of thumb" at this stage is: the drier the material to be ensiled, the smaller the crop particle should be. The coverage of the ensiled matter should be done immediately with plastic sheets (polyethylene). It is very important to use exclusive adequate sheets especially for this purpose. A low quality sheet will permit air penetration and enable mould growth and the further production of mycotoxins, also leading to losses of dry matter and energy content. Once the silo is air tight, respiration stops and fermentation can be initiated. Although not useful in preventive situations due to its highly questionable efficacy, storage length is considered by some authors to have an impact on the mycotoxin content. Richter (2006) reported a decrease in ergot alkaloids produced by Claviceps purpurea during storage. Zearalenone and some of the trichothecenes appear not to be affected by anaerobic and acid conditions in silage (Lepom et al., 1988).
Ensiling time also plays an important role in cases of acetic acid fermentation. Silage is a rich source of nutrients (starch, lactic acid, amongst others) for yeasts and moulds and can therefore become unstable if in contact with oxygen. The aerobic stability can be increased by using appropriate silage additives (heterofermentative bacteria - acetic or propionic acid producers - or organic acids directly applied on the surface contacting the air). The production of acetic acid in the silage begins later than that of lactic acid. This is why it is crucial to wait the appropriate time (at least 4 to 6 weeks) until the heterofermentative bacteria ferment sugars, and, in many cases, part of the lactic acid, into acetic acid. Silages are less stable if they contain residual sugar or starch. Muck and Bolsen (1991) reported a faster growth of yeasts on corn silages. To adequately manage the silo, the silage amount fed each week should guarantee an advance in the silo of 1.0 to 1.5 m and 2.0 to 3.0 m in winter and summer respectively. This advance is related to the design of the silo and therefore its size must be carefully calculated. Another key factor to avoid mycotoxin contamination in the silo is to have a clean-cut face in the feed out phase, as this will give fewer opportunities for the growth of undesired moulds on the surface.
Some take-home messages
Avoiding mycotoxin formation in silages must begin on
Some take-home messages
Avoiding mycotoxin formation in silages must begin on
a. the field, should continue in
b. the silage production process and finalize with
c. the correct management of the open silo.
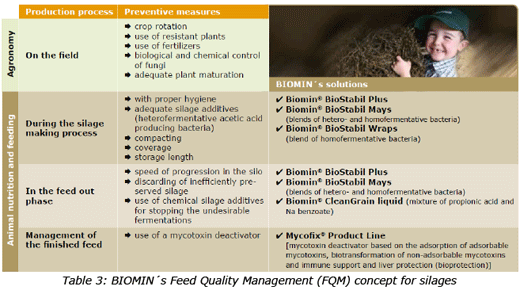
However, in spite of all these prevention steps, already existing mycotoxins will persist in the trough and cause hazardous effects to the animals ingesting them. Therefore, the use of a mycotoxin deactivator is fully justified (Table 3), as a part of the BIOMIN´s Feed Quality Management (FQM) concept. If these measures are taken into account, the probability of producing high quality silage will be increased. BIOMIN´s FQM concept considers the necessity of having different solutions for each chain link in the production process. Well designed product lines such as Biomin® BioStabil and Mycofix® are the result of long term research focused on solving preservation and mycotoxins' troubles on farms. No safe levels of mycotoxins exist since, when dealing with living beings such as plants and animals, many factors have to be taken into account which are impossible to be controlled. Mycotoxin risk management, is possible and should be done not only for silages but for all feedstuffs.
Literature
Diaz DE, Hopkins BA, Leonard LM, Hagler WM, Whitlow LW (2000)
Gratz S and Täubel M (2007)
Jones RK, Duncan HE, Hamilton PB (1981)
Kalac P and Woodford MK (1982)
Kleinmans J (1996)
Lepom P, Baath H, Knabe O (1988)
Losand B (2003)
Muck RE and Bolsen KK (1991)
Ominski KH, Marquardt RR , Sinha RN and Abramson D (1994)
Özsoy S, Altunatmaz K, Horoz H, Kasikci G, Alkan S, Bilal T (2004)
Pelhate J (1977)
Ramakrishna N, Lacey J and Smith JE (1993)
Richter W (2006)
Richter W and Bauer J (1997)
Richter W, Schuster M, Tischner H, Zimmermann G, Doleschel P, Beck R, Lepschy J, Steinhöfel O, Hörügel K, Hanschmann G, Heinze A, Jahn O, Hartung H, Müller G, Thalmann A (2005)
Scudamore KA and Livesey CT (1998)
Schneweis I (2000)
Schrödter R (2004)
Warfield CY, Gilchrist, DG (1999)
Wilkinson, JM (2005)
Yiannikouris A and Jouany JP (2002)
Related topics:
Authors:
DSM-Firmenich
DSM-Firmenich
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.







