Originally published in Research in Veterinary Science 2000, 69, 89–93.
AFLATOXINS (AF), a group of closely related, extremely toxic chemicals, are produced by Aspergillus flavus and Aspergillus parasiticus and can occur as natural contaminants of poultry foods. Aflatoxicosis is an important disease of livestock and poultry. The frequent AF contamination of agricultural commodities and the chronic exposure of poultry to these toxins can mean the difference between profit and loss to the poultry industry (Edds and Bortell 1983, Kaya et al 1990, Leeson et al 1995).
Aflatoxicosis in poultry is characterised by listlessness, anorexia with lowered growth rate, poor food utilisation, decreased weight gain, decreased egg production, and increased susceptibility to environmental and microbial stresses, and increased mortality (Bailey et al 1998, Kubena et al 1998). Also associated with aflatoxicosis is anaemia (Huff et al 1988, Kececi et al 1998), inhibition of immune function (Celik et al 1996, Gabal and Azzam 1998), hepatotoxicosis (Edrington et al 1997, Kiran et al 1998), mutagenesis, teratogenesis, carcinogenesis, and haemorrhage (Edds and Bortell 1983).
Determination of biochemical and haematological toxic effects of AF is important for diagnosis of toxicosis in poultry (Bailey et al 1998, Kececi et al 1998, Ledoux et al 1999). AF toxicity in poultry may be manifested by decreased serum concentrations of total protein, albumin, cholesterol, triglyceride and glucose (Harvey et al 1993, Edrington et al 1997, Kubena et al 1998), uric acid (Abo-Norag et al 1995, Kececi et al 1998), inorganic phosphorus and calcium (Fernandez et al 1994). Broiler chicks given 2·5 to 3·5 mg AF kg–1 diet have shown not only decreased amount of haemoglobin, haematocrit values, thrombocyte counts, percentage of lymphocyte and basophil counts (Huff et al 1988), but also an increased percentage of heterophils (Kececi et al 1998).
Removing AF from contaminated food and foodstuffs remains a major problem and there is a great demand for effective decontamination technology. Decontamination procedures have focused on degrading, destroying, inactivating or removing AF by physical, chemical or biological methods. Large-scale, practical, and cost-effective methods for detoxifying AF-containing foodstuffs are currently not available although a variety of physical, chemical and biological methods for detoxifying AF has been employed with limited success. Therefore, the use of AF-contaminated food remains a significant problem, and one with serious economic implications (Bailey et al 1998, Kubena et al 1998, Ledoux et al 1999, Parlat et al 1999).
Another approach to the problem has been to use nonnutritive and inert adsorbents in the diet to bind AF and reduce their absorption from the gastrointestinal tract. These compounds must not be absorbed from the gastrointestinal tract and must have the ability to bind physically with chemical substances, precluding their absorption. Clays and zeolites have been used for this purpose. These are generally inert and non-toxic to animals (Olver 1997) and have a capacity to bind AF (Phillips et al 1988). The dietary addition of zeolites (Harvey et al 1993, Kececi et al 1998), bentonite(Santurio et al 1999) and hydrated sodium calcium aluminosilicate, HSCAS; a natural phyllosilicate (Kubena et al 1993, 1998, Ledoux et al 1999) and activated charcoal (Edrington et al 1997) have been used for reduction of AF toxicity in chickens.
Clinoptilolite (CLI), a natural zeolite, occurs as laths and plates, many of which display the characteristic tubular morphology typical of basalt-vug heulandite. The laths are commonly 1 to 3 µm in thickness and 5 to 20 µm in length (Ming and Mumpton 1989). When CLI was used in broiler chicks (Oguz and Kurtoglu 2000) and Japanese quail (Parlat et al 1999) for reduction of AF toxicity, significant improvements were observed on AF-related changes in the growth performances of birds. However, no beneficial effect was reported by the addition of CLI to the AF-containing diet (Harvey et al 1993). The present study was undertaken to observe the biochemical and haematological toxic effects of AF in broiler chicks and to determine the preventive efficacy of different levels of CLI (1·5 and 2·5 per cent)·
MATERIALS AND METHODS
Chickens and diet
Three hundred and sixty 1-day-old, unvaccinated broiler chicks (Avian strain) of both sexes were obtained from a commercial hatchery. Individually weighed chicks were divided at random into six groups. There were six replicates of 10 broiler chicks for each dietary treatment, totalling 360 chicks. The chicks were housed in electrically heated batteries under fluorescent lighting and received a commercial basal diet (maize and soybean meal diet 230 g protein, 13·26 MJ ME kg–1) formulated to contain the National Research Council (1994) requirements. Food and water were always available and lighting was continuous. The basal diet was tested for possible residual AF before feeding (Howel and Taylor 1981), and there were no detectable levels present (detection limit 1 µg AF kg–1 food; recovery of the extraction method 95 per cent). The trial period was 3 weeks.
Experimental design
The experimental design consisted of six dietary treatments. (1) CONT: basal diet; (2) AF: basal diet plus 2·5 mg total aflatoxin (AF; the composition given below) kg–1 diet; (3) CLI (1·5 per cent): basal diet plus 15 g clinoptilolite (CLI) kg–1 diet; (4) AF + CLI (1·5 per cent): basal diet plus 2·5 mg AF + plus 15 g CLI kg–1 diet; (5) CLI (2·5 per cent): basal diet plus 25 g CLI; and (6) AF + CLI (2·5 per cent): basal diet plus 2·5 mg aF plus 25 g CLI kg–1 diet. Clinoptilolite (CLI/NUT-1000), which is a member of heulandite-stilbite group, was provided by Incal Biotechnology and Mining Ltd., I· zmir, Turkey; chemical formula is ‘KNa2 Ca2 (Si29AL7 ) O72.32H2 O’.
Aflatoxin
AF was produced on rice by the method of Shotwell et al (1966) with minor modifications by Demet et al (1995). The AF content in rice powder was analysed by the method of Shotwell et al (1966) and measured using thin-layer chromatography (TLC)-densitometry (Perkin Elmer MPF 43-A) on TLC spots. The AF within the rice powder consisted of 76·40 per cent AFB1 , 16·12 per cent AFB2 , 6·01 per cent AFG1 and 1·47 per cent AFG2 based on total AF in the rice powder (detection limit: 1 µg AF kg–1 rice powder; recovery of the extraction method: 92 per cent). The rice powder was incorporated into the basal diet to provide the desired amount of 2·5 mg AF kg–1 food.
Serum biochemical and haematological analysis
When the chicks reached 3 weeks of age, the feeding trial was terminated and 10 broilers from each treatment were selected at random and bled by cardiac puncture. Serum concentrations of total protein, albumin, inorganic phosphorus, calcium, uric acid, total cholesterol and glucose were determined on a clinical chemistry analyser (Gilford Impact 400E, Gilford Systems, OH, USA). The red blood cell (RBC), white blood cell (WBC) and thrombocyte counts were determined by a haemocytometer method using Natt–Herrick solution; haematocrit values were measured by the microhaematocrit method. Haemoglobin amounts were determined by the cyanmethaemoglobin method; the mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) were calculated; differential leukocyte counts were determined as described by Konuk (1975).
Statistical analysis
The data for serum biochemical and haematological values were grouped and expressed as mean ± pooled standard errors of means. The results obtained were statistically analysed using Duncan’s multiple range test (SPSS 1988). Statements of statistical significance are based on P < 0·05.
RESULTS
Feeding AF alone caused significant decreases in the serum levels of total protein, albumin, inorganic phosphorus, uric acid and total cholesterol (Table 1). The addition of CLI (1·5 per cent) to the AF-containing diet provided a partial improvement in serum albumin, inorganic phosphorus, uric acid and total cholesterol levels. This intermediate improvement was also seen in serum inorganic phosphorus and total cholesterol values in chicks fed on a diet containing AF plus CLI (2·5 per cent) addition to the AF-containing diet. There were no significant differences in calcium and glucose levels with the treatments.
The AF-alone group showed a significant decrease in RBC counts, haematocrit values, MCV, haemoglobin, thrombocyte counts, and percentage of lymphocyte counts (Tables 2 and 3). The decrease in monocyte counts was not significant compared with the controls. In addition, WBC and percentage of heterophil counts increased in chicks given AF-alone. There were no significant differences in MCH, MCHC, and percentage of basophil and eosinophil counts with these treatments. The addition of CLI (1·5 per cent) to the AF-containing diet significantly improved the decrease in thrombocyte counts caused by AF. The AF-related changes in RBC counts, haematocrit, haemoglobin, WBC, percentage of lymphocyte and monocyte counts were intermediately ameliorated by adding of CLI (1·5 per cent) to the AF-containing diet.
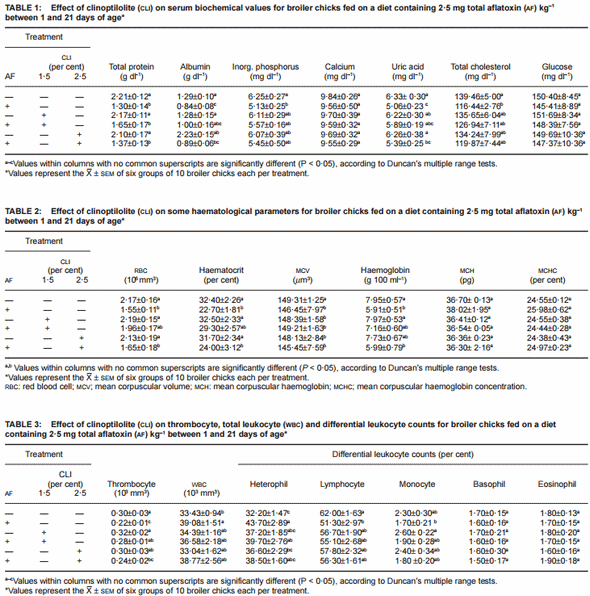
The addition of CLI (both 1·5 and 2·5 per cent) into the AFfree diets did not significantly alter the serum biochemical and haematological values compared with the controls, except the decline on MCV counts.
DISCUSSION
Chronic aflatoxicosis may be diagnosed by determining serum biochemical and haematological alterations before clinical symptoms become apparent (Kececi et al 1998). The use of agents that act as antidotes or antagonise the effects of toxic substances such as AF have therapeutic and economic importance. The major advantages of these absorbents include expense, safety and easy administration through addition to animal foods (Ledoux et al 1999). In this study, neither serum biochemical nor haematological values were negatively affected by the addition of CLI (both 1·5 and 2·5 per cent) to the AF-free diet, except MCV values. These data show that CLI used in this study was inert and non-toxic as indicated by Olver (1997) and Parlat et al (1999).
In this study, experimental aflatoxicosis was induced in broiler chicks by feeding 2·5 mg total AF kg–1 diet from 1 day for 3 weeks. In intoxicated broilers, significant decreases in serum total protein and albumin levels were found (P < 0·05), in agreement with findings in approximately21-day-old chicks (Kubena et al 1993, Edrington et al 1997, Bailey et al 1998, Kececi et al 1998). Kubena et al (1990) reported that decreased serum total protein and albumin in broilers as the results of AF were not alleviated by some HSCAS, but were completely (Kubena et al 1998, Ledoux et al 1999) or partially (Abo-Norag et al 1995) improved by the addition of different types of HSCAS in broiler chicks given AF (3·5 to 5 mg kg–1 diet).
AF may cause alteration of calcium and inorganic phosphorus metabolism (Glahn et al 1991). In the present study, a significant decrease was found in serum inorganic phosphorus level (P < 0·05), in agreement with the findings of other studies (Harvey et al 1993, Ledoux et al 1999), but no significant difference in serum calcium with either AF or other additions. The decreased inorganic phosphorus of chicks given AF was partially improved by the dietary addition of two doses (1·5 and 2·5 per cent) of CLI (P > 0·05). A significant decrease in serum uric acid was seen in chicks given AF-alone (P < 0·05) as expected (Huff et al 1986, Kececi et al 1998). In the present study, the uric acid levels in chicks fed a diet with AF plus CLI (1·5 per cent) were close to those seen with the control diet as seen with 0·5 per cent HSCAS (Abo-Norag et al 1995) and 0·5 per cent zeomite (a natural zeolite; Harvey et al 1993). In this study, decreased serum cholesterol in chicks given AF (2·5 mg kg–1) is consistent with others, but while Kececi et al (1998) reported a significant improvement in the decreased serum total cholesterol levels by the dietary addition of bentonite (0·5 per cent) to the AF-containing diet (P < 0·05) and Ledoux et al (1999) by the addition of a special HSCAS-type in broiler chicks. In the present study the addition of CLI (1·5 and 2·5 per cent) to the AF-containing diet provided only a partial improvement in serum total cholesterol levels of chicks (P > 0·05).
In this study, the decreases in the mean values of haematocrit, haemoglobin, RBC, MCV, thrombocyte counts and percentage of lymphocyte counts in AF-fed chicks indicate the depressing effect of AF on haemopoietic tissue as indicated by others (Campbell et al 1983, Huff et al 1986, 1988, Kubena et al 1990, Kececi et al 1998). Oguz and Kurtoglu (2000) provided significant improvements in growth performances of chicks by the dietary addition of CLI (1·5 per cent) to the AF (2·5 mg kg–1)-containing diet in agreement with the findings of this study. However, Harvey et al (1993) reported no beneficial alleviation by the addition of CLI (0·5 per cent) to the AF (3·5 mg kg–1)-containing diet. This difference might have resulted from the type or dose of CLI or level of AF in food.
A significant improvement was observed in thrombocyte counts by the addition of CLI (1·5 per cent) to the AF-containing diet in the present study (P < 0·05). There was an intermediate alleviation in some serum biochemical and haematological parameters by the addition of CLI (1·5 per cent) to the AF-containing diet (P > 0·05). The preventive effect of low-level CLI (1·5 per cent) used in this study was more effective than the high-level CLI (2·5 per cent) against the toxic effect of AF. Although the protective effect of CLI against the toxic effects of AF on investigated variables was not as great as might have been predicted, these improvements should contribute to a solution of the AF problem in poultry
ACKNOWLEDGEMENTS
The authors gratefully acknowledge TUBITAK (Veterinary Medicine and Animal Husbandry Research Grand Committee), which funded this project. Project no: VHAG-1437.
REFERENCES
ABO-NORAG, M., EDRINGTON, T.S., KUBENA, L.F., HARVEY, R.B. & PHILLIPS, T.D. (1995) Influence of hydrated sodium calcium aluminosilicate and virginiamiycin on aflatoxicosis in broiler chicks. Poultry Science 74, 626–632
BAILEY, R.H., KUBENA, L.F., HARVEY, R.B., BUCKLEY, S.A. & ROTTINGHAUS, G.E. (1998) Efficacy of various inorganic sorbents to reduce the toxicity of aflatoxin and T-2 toxin in broiler chickens. Poultry Science 77, 1623–1630
CAMPBELL, M.L., MAY, J.D., HUFF, W.E. & DOERR, J.A. (1983) Evaluation of immunity of young broiler chickens during simultaneous aflatoxicosis and ochratoxicosis. Poultry Science 62, 2138–2144
CELIK, I., DEMET, O., DONMEZ, H.H., OGUZ, H. & BOYDAK, M. (1996) Determination of phagocytic and candidacidal activities of peritoneal macrophages isolated from chickens fed with aflatoxin and an aflatoxin adsorbing agent, polyvinylpolypyrrolidone. Veteriner Bilimleri Dergisi 12, 145–151
DEMET, O., OGUZ, H., CELIK, I. & ADIGUZEL, H. (1995) Production of aflatoxin on wheat, corn, rice and peanut. Veteriner Bilimleri Dergisi 11, 135–140
EDDS, G.T. & BORTELL, R.A. (1983) Biological effects of aflatoxins: poultry aflatoxin and Aspergillus flavus in corn. In: Bulletin of the Alabama Agricultural Experiment Station. Eds U.L. Diener, R.L. Asquit, J.W. Dickens. Alabama, AL: Auburn University, pp. 64–66
EDRINGTON, T.S., KUBENA, L.F., HARVEY, R.B. & ROTTINGHAUS, G.E. (1997) Influence of superactivated charcoal on the toxic effects of aflatoxin or T- 2 toxin in growing broilers. Poultry Science 76, 1205–1211
FERNANDEZ, A., VERDE, M., GASCON, M., RAMOS, J., GOMEZ, J., LUCO, D.F. & CHAVEZ, G. (1994) Variations of clinical, biochemical parameters of laying hens and broiler chickens fed aflatoxin-containing feed. Avian Pathology 23, 37–47
GABAL, M.A. & AZZAM, A.H. (1998) Interaction of aflatoxin in the feed and immunisation against selected infectious diseases in poultry. II. Effect on one-day-old layer chicks simultaneously vaccinated against Newcastle disease infectious bronchitis and infectious bursal disease. Avian Pathology 27, 290–295
GLAHN, R.P., BEERS, K.W., BOTTJE, W.G., WIDEMAN, R.F., HUFF, W.E. & THOMAS, W. (1991) Aflatoxicosis alters avian renal function, calcium, and vitamin D metabolism. Journal of Toxicology and Environmental Health 34, 309–321
HARVEY, R.B., KUBENA, L.F., ELLISALDE, M.H. & PHILLIPS, T.D. (1993) Efficacy of zeolitic ore compounds on the toxicity of aflatoxin to growing broiler chickens. Avian Diseases 37, 67–73
HOWEL, M.V. & TAYLOR, P.W. (1981) Determination of aflatoxins, ochratoxin A, and zearalenone in mixed feeds, with detection by thin layer chromatography or high performance liquid chromatography. Journal of the Association Official Analytical Chemistry 64, 1356–1363.
HUFF, W.E., KUBENA, L.F., HARVEY, R.B., PHILLIPS, T.D., HAGLER, W.M., SORENSON, S.P., PHILLIPS, T.D. & GIEGLER, C.R. (1986) Individual and combined effect of aflatoxin and deoxynivalenol (DON, Vomitoxin) in broiler chicken. Poultry Science 65, 1291–1298
HUFF, W.E., HARVEY, R.B., KUBENA, L.F. & ROTTINGHAUS, G.E. (1988) Toxic synergism between aflatoxin and T-2 toxin in broiler chicken. Poultry Science 67, 1418–1420
KAYA, S., YAVUZ, H. & AKAR, F. (1990) Mycotoxin residues in some oily seed meals. A.U. Veteriner Fakultesi Dergisi 37, 173–180
KECECI, T., OGUZ, H., KURTOGLU, V. & DEMET O. (1998) Effects of polyvinylpolypyrrolidone, synthetic zeolite and bentonite on serum biochemical and haematological characters of broiler chickens during aflatoxicosis. British Poultry Science 39, 452–458
KIRAN, M.M., DEMET, O., ORTATATLI, M. & OGUZ, H. (1998) The preventive effect of polyvinylpolypyrrolidone on aflatoxicosis in broilers. Avian Pathology 27, 250–255
KONUK, T. (1975) Pratik Fizyoloji-l. Ankara: Ankara Universitesi Veteriner Fakultesi, A.U. Basimevi, No: 314
KUBENA, L.F., HARVEY, R.B., HUFF, W.E. & CORRIER, D.E. (1990) Efficacy of hydrated sodium calcium aluminosilicate to reduce the toxicity of aflatoxin and T- 2 toxin. Poultry Science 69, 1078–1086
KUBENA, L.F., HARVEY, R.B., PHILLIPS, T.D. & CLEMENT, B.A. (1993) Effects of hydrated sodium calcium aluminosilicate on aflatoxicosis in broiler chicks. Poultry Science 72, 651–657
KUBENA, L.F., HARVEY, R.B., BAILEY, R.H., BUCKLEY, S.A. & ROTTINGHAUS, G.E. (1998) Effects of hydrated sodium calcium aluminosilicate (TBind™) on mycotoxicosis in young broiler chickens. Poultry Science 77, 1502–1509
LEDOUX, D.R., ROTTINGHAUS, G.E., BERMUDEZ, A.J., ALANSO-DEBOLT, M. (1999) Efficacy of hydrated sodium calcium aluminosilicate to ameliorate the toxic effects of aflatoxin in broiler chicks. Poultry Science 78, 204–210 92
H. Og?uz, T. Keçeci?, Y. O. Bi?rdane, F. Önder, V. Kurtog?lu LEESON, S., DIAZ, G. & SUMMERS, J.D. (1995) Aflatoxins. In: Poultry Metabolic Disorders and Mycotoxins. Eds S. Leeson, G. Diaz, J.D. Summers. Ontario, Canada: University Books, pp. 248–279
MING, D.W. & MUMPTON, F.A. (1989) Zeolites in soils. In: Minerals in Soil Environments. 2nd edn. Madison, WI: Soil Science Society of America, Book series, No. 1. pp 873–911
NATIONAL RESEARCH COUNCIL (1994) Nutrient Requirements of Poultry, 9th edn, Washington DC: National Academy Press, pp. 44–45 OGUZ, H. & KURTOGLU, V. (2000) Effect of clinoptilolite on performance of broiler chickens during experimental aflatoxicosis. British Poultry Science (in press)
OLVER, M.D. (1997) Effect of feeding clinoptilolite (zeolite) on the performance of three strains of laying hens. British Poultry Science 38, 220–222
PARLAT, S.S., YILDIZ, A.O. & OGUZ, H. (1999) Effect of clinoptilolite on fattening performance of Japanese quail (Coturnix Coturnix Japonica) during experimental aflatoxicosis. British Poultry Science 40, 495–500
PHILLIPS, T.D., KUBENA, L.F., HARVEY, R.B., TAYLOR, D.R. & HEIDELBAUGH, N.D. (1988) Hydrated sodium calcium aluminosilicate. A high affinity sorbent for aflatoxin. Poultry Science 67, 243–247
SANTURIO, J.M., MALLMANN, C.A., ROSA, A.P., APPEL, G., HEER, A., DAGEFORDE, S. & BOTTCHER, M. (1999) Effect of sodium bentonite on the performance and blood variables of broiler chickens intoxicated with aflatoxin. British Poultry Science 40, 115–119
SHOTWELL, O.L., HESSELTINE, C.V., STUBBLEFIELD, R.D. & SORENSON, W.G. (1966) Production of aflatoxin on rice. Applied Microbiology 14, 425–429
SPSS (1988) SPSS/PC+V.2·0. Base Manual for the IBM PC/XT/AT and PS/2. Marija and Morusis. Chicago, IL: Soil Science Society of America, Inc.