Avian Mycotoxicosis in Developing Countries
Published: September 18, 2014
By: Adeniran Lateef Ariyo, Ajagbonna Olatunde Peter, Sani Nuhu Abdulazeez and Olabode Hamza Olatunde (University of Abuja)
1. Introduction
Avian mycotoxicosis refers to all the diseases caused by the effect of mycotoxin in birds. These diseases may not be pathognomonic and sometimes subclinical and difficult to diagnose. The problem is worldwide but effort will be made to localise the effect of these diseases in developing countries. Developing countries have more than 50% of total meat and egg production in global poultry market: [1]. The global poultry meat market is 86.8 million tonnes, consisting of chicken: 85.6%, turkey: 6.8%; duck: 4.6%; goose and guinea fowl: 2.6%.
Table 1. Development of poultry meat production in developed and developing countries (million tonnes).
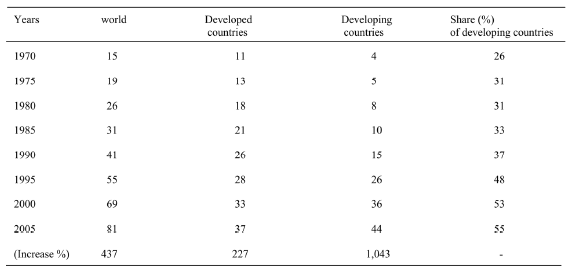
Table 2. Development of poultry hen egg production in developed and developing countries (million tonnes)
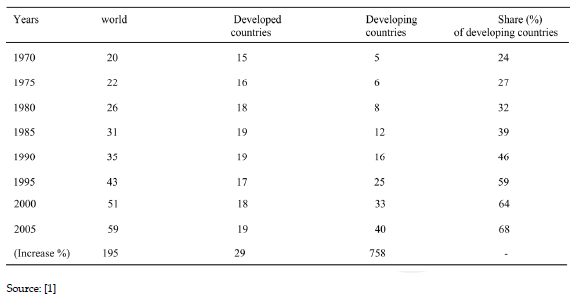
The world market poultry meat and egg market is been influenced by the production and managemental style from the developing countries. Avian mycotoxicosis is a great constraint in poultry industry, because the disease is characterized by immunosuppresion, hepatotoxicity, nephrotoxicity, loss of egg production, mutagenicity and tetratogenicity.
Mycotoxin are antinutritive factor present in feed ingredients and in concentrated feed, they are a group of secondary fungal metabolites of low molecular weight, diverse and ambiguous in nature, which are specifically implicated in causing toxic effect in animals and man [2,3]. Mycotoxicosis has been a major but unrecognized food safety issue for several centuries. They are naturally occurring contaminants that causes health related problems when it gets into the body through natural route of ingestion, inhalation or may be absorbed through the skin [4]. They are endogenously generated in foods as a result of secondary metabolism [5]. These metabolites are synthesized in or on food surfaces and transported through the food chain[6]. Mycotoxin production takes place in the mycelium after active fungal growth, but may accumulate in specialized structures such as sclerotia, conidia or in surrounding area [7]. Animal studies have shown that, besides acute effects, mycotoxins can cause carcinogenic, mutagenic and teratogenic effects. Mycotoxinscontaminated poultry feed can lead to the transfer of toxins through meat and egg to human beings.
The Food and Agriculture Organization [8] estimating that as much as 25% of the world’s Agricultural commodities are contaminated with mycotoxins, leading to significant economic losses. Mycotoxigenic fungi genera include; Aspergillus, Penicillium and Fusarium. The important mycotoxin in the developing countries include aflatoxins, ochratoxins, citrinin, T-2 toxin, deoxynivalenol (DON), fumonisins and zearalenone.
Table 3. Showing fungi genera and the associated mycotoxin.

2. Avian aflatoxicosis
2.1. Aetiology
Avian aflatoxicosis is a disease of poultry caused by aflatoxin. Aflatoxins (AF) are widely distributed toxins produced by Aspergillus. Of the over 180 species of Aspergillus, only a few are aflatoxigenic. After the discovery of AF in the 1960s, A. flavus and A. parasiticus of the section Flavi were the only known AF producers producing the B and B/G types of AF, respectively [10]. Other aflatoxigenic species that subsequently emerged are A. nomius (B and G types), A. bombycis (B and G AF), A. ochraceoroseus, and A. pseudotamarii (B type), but they occur less frequently [11,12]. A. tamarii, A. parvisclerotigenus (B types), A. rambellii and certain members of Aspergillus subgenus Nidulantes namely: Emericella venezualensis [13] and E. astellata [14] have now been included in the growing list of aflatoxigenic species. A. arachidicola sp. Nov. and A. minisclerotigenes sp. Nov that produce both forms of the toxin, are the latest emerging aflatoxigenic species. The unexpected new comer is A. niger, an ochratoxin (OT) producer which was discovered over four decades ago but was never associated with AF synthesis. However, in a search for aflatoxigenic fungi in Romanian medicinal herbs, [15] showed the capacity of some strains of A. niger to produce AFB1 [16].
AFBI is a member of aflatoxin group is one of the most carcinogenic natural product formed in nature [17]. AF has been detected in most countries of the world. Four toxins soon identified: Aflatoxin B1, B2, G1, G2-blue or green florescence under UVlight.• AflatoxinB1most important -highly carcinogenic and widespread occurrence in foods •(B1> M1> G1> B2> M2~ G2). Aflatoxin M1: hydroxylated product of B1appears in milk, urine, and feces as metabolic product
2.2. Factor enhancing AF prevalence in feed ingredients and poultry feed
There are several factors enhancing the prevalence of aflatoxigenic fungi and aflatoxin production in developing countries. The factor include the following:
The food materials must be infected by aflatoxigenic fungi which deposit the toxins on feed ingredients and concentrated feeds.
The substrate which may be feed ingredients and concentrated feeds posseses a source of energy in the form of carbohydrates and organic and inorganic source of nitrogen, trace elememts and moisture for growth of mould and toxin production [8].
Among cereal, the size and integrity of the seed coat also affects the susceptibility of fungal infection and mycotoxin formation [18]
The environmental condition favouring mould growth and AF production are hot and humid conditions, the optimal temperature varies between 240Cand 280C [19] and seed moisture content of at least 17.5% [20]. These conditions that favours mould growth are present in most developing countries.
Soil type also affect the level of AF contamination of crops for example, light sandy soil support rapid growth of fungi [21]
Presence of other microorganism either bacteria for example presence of Streptococcus lactis and Lactobacillius casei causes reduction in AF production by A. parasiticus [20]. Meanwhile fungal metabolites like rubratoxin and cerulenin enhances AF production [16].
Agricultural practices also affect AF contamination of feed ingredient. Off season harvesting and harvesting system that enhances seed breakage would also increase the degree of AF production [22].
A well aerated storage condition used in most developing country to store feed ingredients increases metabolism and subsequent AF production [23].
2.3. Occurrence of AF
AF has been found as contaminants in animal feed ingredient worldwide. The occurrence in developing countries is more because there is no strict food and feed quality control programmes to reduce the burden of AF. Also their environmental condition presented as hot and humid climate makes most developing country vulnerable to AF in poultry feed. Among the four AF that are of significance in poultry include; AFB1, B2, G1and G2. AFB1 was detected mostly from animal and feed ingredients from developing countries. Among the feeds ingredients, sorghum, wheat, maize were the most investigated, data on groundnut cake, cotton seed meal and fish meal showed high level of AF contamination. The highest level of AFB1 contamination of feed ingredient were reported in corn from Pakistan 25μg/kg [24], Nigeria wheat was found to be contaminated with 17.10-20.53 μg /kg [25].
Higher level of contamination of AF were found in the animal feed than the feed ingredient possibly because of the storage condition which allowed growth and proliferation of mycotoxigenic fungi. In Nigeria AF was detected in poultry feed at 0.0-67.9 μg/kg [26], wheat 17.10-20.53 μg/kg [25], millet 1370-3475 μg/kg [23].
AF contamination of feed and feed ingredients has been a major concern in many developing country like Pakistan where concentration ranging from 24-37.62 μg/kg were found in poultry feed and poultry feed ingredient [24].
Table 4. Showing occurrence of AF in animal feed and feed ingredients in developing countries
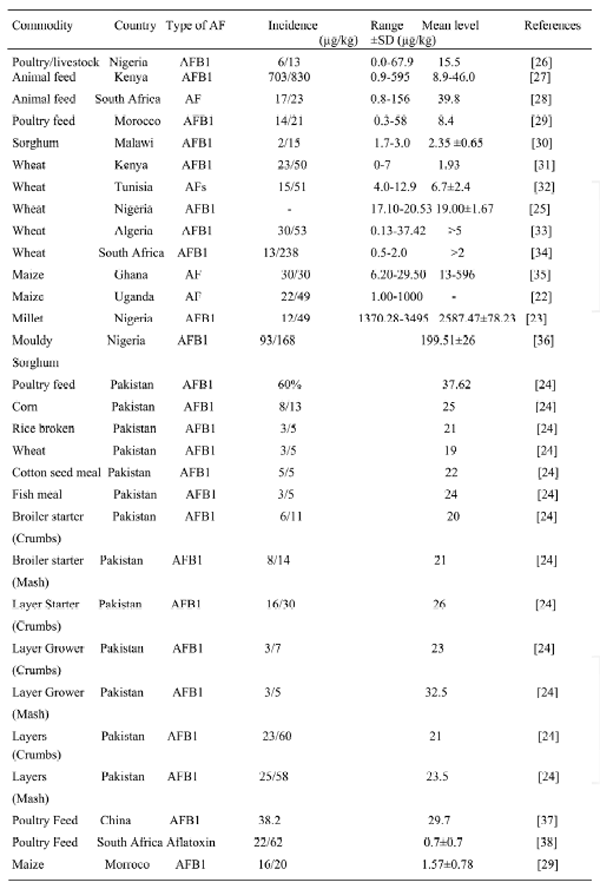
An unacceptably high level of concentration of AF that ranges from 1-1000 μg/kg was found in maize from Uganda[22] About 70% of wheat samples investigated in Algeria were contamination at a range of 0.13-3742mg/kg [33]
Table 5. Main clinical signs, performance and pathological features in food producing animals exposed to AF in selected studies.
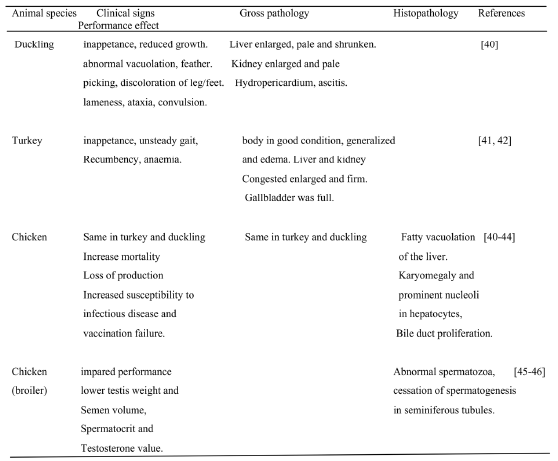
2.4. Pharmacological interaction
Aflatoxicosis has effect on plasma half-life, thus it affects drug effect in the body. [47] observed that chlortetracycline plasma concentrations were lowered due to decreased drug binding to plasma protein [48]. Though opinion differed considerably on sparing or aggravating effect caused by the addition of chlortetracycline to feed contaminated with aflatoxin [49,50].
2.5. Metabolism and residues
In broilers, metabolites of aflatoxins BI and B2 concentrated in kidney and liver but cleared within 4 days. Then metabolism of Aflatoxin B1 into conjugated aflatoxins B2a and Ml occurred in the liver, which will be metabolized to aflatoxicol [51-54]. Aflatoxin BI was excreted in the bile, urine, and feces as 6 major metabolites [55]. The half-life of aflatoxin BI in laying hens is about 67 hours [56], though feed: egg transmission is about 5000:1 [57]. Most aflatoxin excreted through the bile and intestine, but aflatoxin BI and aflatoxicol were detected in ova and eggs for 7 days or longer [58-59]. Aflatoxin BI accumulated in reproductive organs and its subsequent transmission to eggs and hatched progeny (yolk sac and liver) in poultry[60]. It is well established that AFB1 is both carcinogenic and cytotoxic. For example, synthesis of both RNA and DNA was inhibited when AF (5mg/kg of feed) was given to rats over a 6-week-period. The activated AFB1 metabolite (i.e. AFB1-8, 9-epoxide) forms a covalent bond with the N7 of guanine [61] and forms AFB1-N7-guanine adducts in the target cells. The results are G_T transversions, DNA repair, lesions, mutations and subsequently tumor formation [57]. The reactive epoxide can also be hydrolyzed to AFB1-8, 9-dihydrodiol which ionizes to form a Schiff’s base with primary amine groups in the proteins [58]. The short-lived epoxide AFB1 has also been associated with coagulopathy due to reduced synthesis of vitamin K and other clotting factors as a result of sub-lethal intoxication of animals [62]. With regard to the cytotoxic effects, AFB1 has been shown to induce lipid peroxidation in rat livers leading to oxidative damage to hepatocytes [63]. A more recent study [64], has demonstrated that AFB1 can inhibit cyclic nucleotide phosphodiesterase activity in the brain, liver, heart, and kidney tissues.
3. Avian ochratoxicosis
3.1. Aetiology
This is a disease of bird caused by Ochratoxins. Ochratoxins are among the most toxic mycotoxins to poultry. They are nephrotoxins found in grains and feeds worldwide [65- 66]. Ochratoxins are isocoumarin compounds linked to L-b-phenylalanine and are designated A, B, C, and D, because of their methyl and ethyl esters. Ochratoxin A (OTA) is the most common and most toxic, and is relatively stable. OTA production is dependent on different factors such as temperature, water activity (aw) and medium composition, which affect the physiology of fungal producers. In cool and temperate regions, OTA is mainly produced by Penicillium verrucosum [67, 68] or P. Nordicum [69, 70]. P. verrucosum mainly contaminates plants such as cereal crops, whereas P. nordicum has been mainly detected in meat products and cheese [69]. In tropical and semitropical regions, OTA is mainly produced by Aspergillus ochraceus [71-73]. A. ochraceus is also referred to as A. allutaceus var allutaceus [71]. A. ochraceus have been reported in a large variety of matters like nuts, dried peanuts, beans, spices green coffee beans and dried fruits, but also in processed meat and smoked and salted fish [71]. Two other species of Aspergillus section nigri, A. niger var niger [74-75] and A. carbonarius[76,77] have been reported as OTA producers. The OTA contamination of substrate such as cereals, oilseeds and mixed feeds in warm zones is thought to be due to A.niger var niger in addition to A. ochraceus species [78], whereas A. carbonarius seems to be more common in grapes, raisins and coffee [79-80].
Recently, [81] isolated two new OTA producing Aspergillus species from coffee beans. These species, A. lacticoffeatus and A. sclerotioniger, need further investigations and are provisionally accepted in section Nigri. In addition, another Aspergillus species, A. alliaceus also named Petromyces alliaceus isolated from onions [82] has been previously reported as OTA producer under laboratory conditions [83]. This species has been suspected to be responsible for the occasional OTA contamination in Californian figs [84, 85] an Argentinean medicinal herbs [67]. The biosynthetic pathway for OTA has not yet been completely established. However, labeling experiments using both 14C- and 13C-labelled precursors showed that the phenylalanine moiety originates from the schikimate pathway and the dihydroisocoumarin moiety from the pentaketide pathway. The first step in the synthesis of the isocoumarin polyketide consists in the condensation of one acetate unit (acetyl-CoA) to four malonate units. Recent data showed that this step requires the activity of a polyketide synthase [86]. Moreover, the gene encoding polyketide synthase appears to be very different between Penicillium and Aspergillus species [86]. In A. ochraceus, the gene of polyketide synthase is expressed only under OTA permissive conditions and only during the early stages of the mycotoxin synthesis [86]. No such data are presently available on penicillium. In Penicillium species, ‘ [86]’ observed that P.nordicum and P. verrucosum use two different polyketide synthases for OTA synthesis. This difference is probably related to the P. verrucosum ability to produce citrinin, also a polyketide-based mycotoxin, in addition to OTA.
Table 6. Showing the occurrence of Ochratoxin in developing countries in a selected surveys.
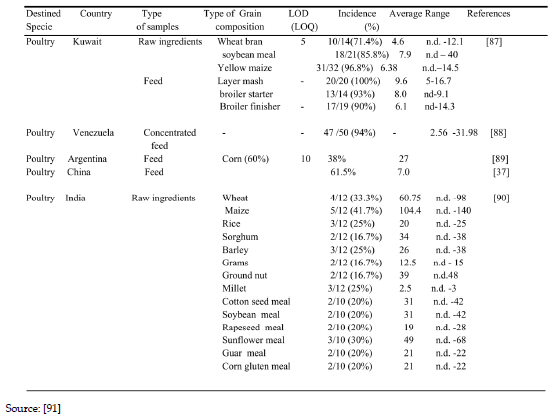
3.2. Occurrence of ochratoxin
OTA was reported by [90] in raw ingredient for making poultry feed were found to be contaminated at range of 00-140 μg/kg in maize 00-98mg/kg in wheat followed by sunflower meal at 00-68 μg/kg. The incidence in India ranges from 16-41% [90]. Also high level of contamination was reported in soya meal from Kuwait n-d-40 μg/kg [87]. [88] reported a contamination level of 2.56-31.98 μg/kg in Venezuela concentrated poultry feed investigated.
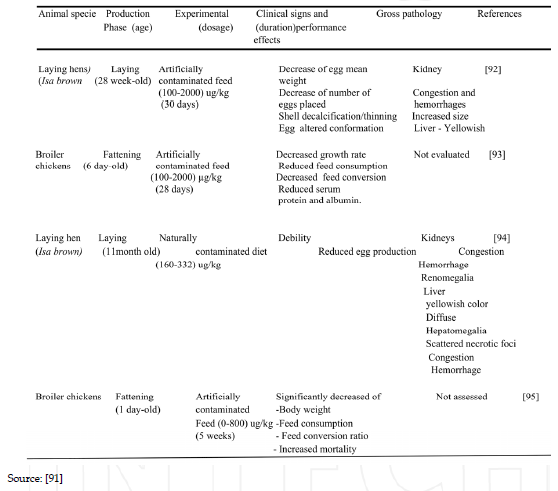
Table 7. Showing the main clinical signs, performance and pathological features in poultry exposed to OTA in selected studies
4. Funmonism
4.1. Aetiology
The fumonism, are food borne carcinogenic mycotoxin that affect animals and man. They are evaluated as group 2B component carcinogen [96]. It has various analog. Twenty eight fumonisin analogs separated into four main groups, as fumonisin A, B, C, and P series has been identified. The fumonisin B (FB) analogs, comprising toxicologically important FB1, FB2, and FB3, are the most abundant naturally occurring fumonisins, with FB1 predominating and usually being found at the highest levels [96] in feed ingredients and poultry feed in world wide. FB1 accounts for about 80% of the total fumonisins produced inthis substrates, while FB2 usually makes around 20% and FB3 usually makes up from about 5% when cultured on corn or rice or in liquid medium [97-100].
Different Fusarium species have been reported to produce fumonisins
4.2. Occurrence of fumonisin
FB1 a potent carcinogen was found in maize investigated from developing countries like Argentina, Benin, Egypt, Nepal, Honduras, Malawi, Zambia Botswana, and Tanzania at a range between 35- 65,000 μg/kg. [101-109]. Poultry feed investigated in china was contaminated with 1854.3mg/kg(37).
No doubt the climatic condition to the agricultural practices in these country allow the growth of fungi and subsequent elaboration of toxin in their substrate.
Table 8. Occurrence of FB1 In Feed Ingredient In Developing Countries
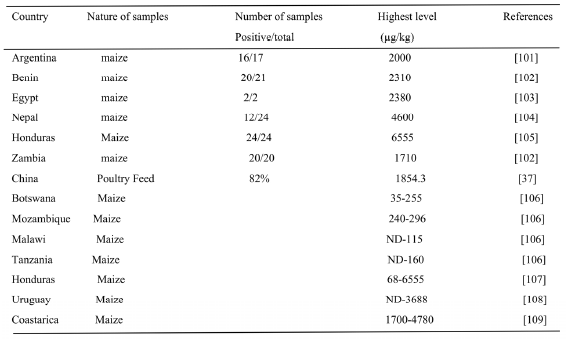
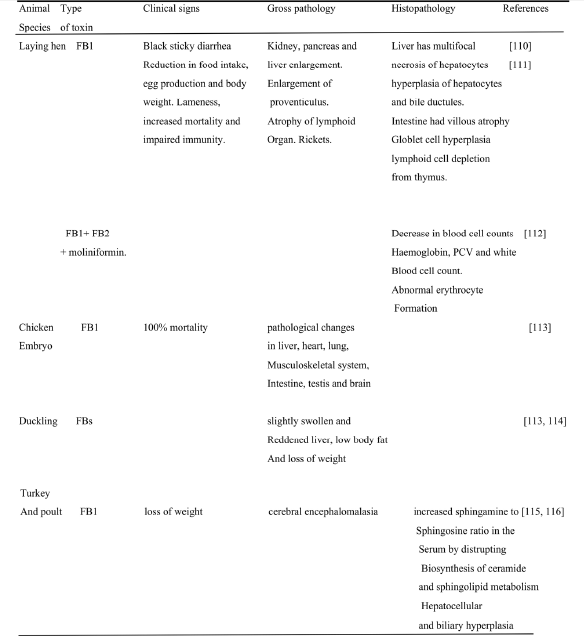
Table 9. Showing the main clinical signs, performance and pathological features in poultry exposed to fumonism in selected studies
5. Fusariotoxin poisoning
Synonyms Fusariomycotoxicosis, trichothecene mycotoxicosis, T-2 toxicosis, vomitoxicosis, zearalenone toxicosis. They are responsible for various diseases of birds in developing countries.
5.1. Aetiology
The trichothecenes include deoxynivalenol (DON), 3, monoacetyldeoxynivalenol (3- AcDON), 15, mono-acetyldeoxynivalenol (15-AcDON), nivalenol (NIV), HT-2 toxin (HT-2), neosolaniol (NEO), T-2 toxin (T-2), T-2 tetraol and T-2 triol, diacetoxyscirpenol (DAS), MASmonoacetoxyscirpenol (MAS) and fusarenone-X.
Different fungi species of the general Fusarium are responsible for the production of this group of mycotoxins. Major producers of trichothecenes are F. graminearum, F. culmorum, F. cerealis, F. poae.
5.2. Occurrence of trichothecenes
Occurrence of trichothecenes in feed ingredients and poultry feed in developing countries is as a result of ubiquitous nature of the fungi which are generally found when certain cereal crops like maize, wheat, corn, millet where grown under stressful condition such as drought. These mould occur in soil, hay and especially grains undergoing microbial and possibly enzymatic degradation [20]. Direct contamination or indirect contamination of feed may occur. Direct contamination occur when the poultry feed were infected with mycotoxigenic fungi.
Indirect contamination may be as a result of fungi elaborating its toxin into the substrate, the incriminating fungi may be removed but the toxin remained in the poultry feed made from such feed ingredients.
Since moulded feed are part of the diet of animal in developing counties, thus all poultry feed are suspect and may contain different level of Trichotheceus toxin.
DON has been reported in maize from Nigeria, South Africa, Argentina, Brazil Pakistan at different concentration that ranges from 0.05-2650 mg/kg [116-120].
6. Diagnosis
Diagnosis is made through observing the appropriate field signs, finding gross as well as microscopic tissue lesions, and detecting the suspected toxin in grains, forages, or the ingesta of affected animals. However, the tests required to detect these toxins are complex and few diagnostic laboratories offer tests for multiple trichothecenes in developing countries. The samples of choice include both refrigerated and frozen carcasses for necropsy examination and a representative sample of the suspected contaminated grain source. Because the toxin is produced under cold conditions, the grain sample should be frozen rather than refrigerated for shipment to the diagnostic laboratory.
Table 10. Occurrence of Trichothecenes in Feed Ingredients In Developing Countries
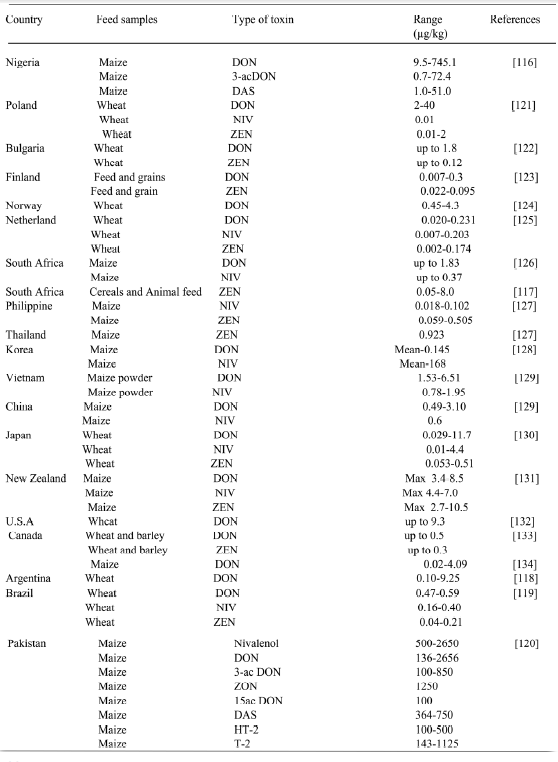
Table 11. Showing the main clinical signs, gross and histopathological changes in some poultry species.
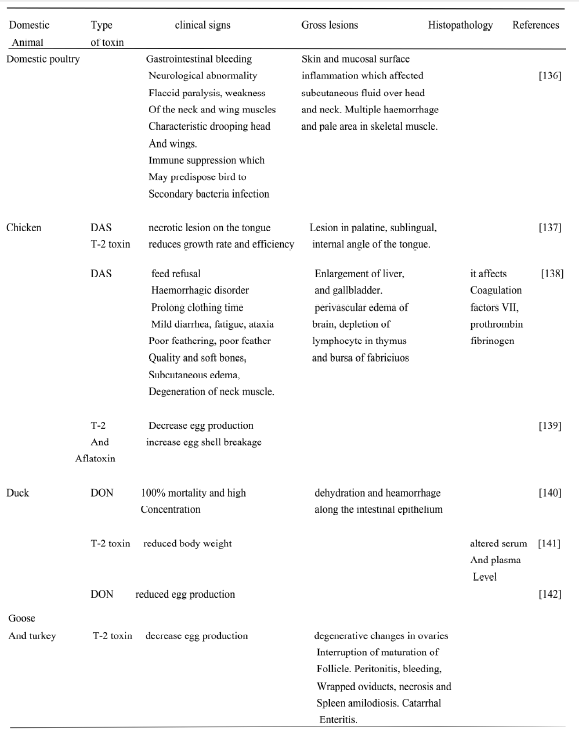
7. Management of avian mycotoxicosis
This involves various practises that reduces fungal contamination of feedstuff and possible use of mycotoxin binders in feed. The clinical signs seen in avian mycotoxicosis are not pathognomonic so the presence of toxin in feed with the associated clinical signs may help clinician to make effective diagnosis and withdrawal of contaminated feed can help to ameliorated the disease condition. Preventive and control of mould in feed is key to achieving control of avian mycotoxicosis.
Following good agricultural practices during both pre-harvest and post-harvest conditions would, minimize the problem of contamination by mycotoxins such as aflatoxins, ochratoxin and trichothecene mycotoxins. These include appropriate drying techniques, maintaining proper storage facilities and taking care not to expose grains.
7.1. Prevention and control of mycotoxins in stored grains and seeds
7.1.1. Dry the feed ingredients
Fungi cannot grow or mycotoxins be produced in properly dried foods, so efficient drying of commodities and maintenance of the dry state is an effective control measure against fungal growth and mycotoxin production.
To reduce or prevent production of most mycotoxins, drying should take place as soon after harvest and as rapidly as feasible. The critical water content for safe storage corresponds to a water activity (aw) of about 0.7. Maintenance of feeds below 0.7 aw is an effective technique used throughout the world for controlling fungal spoilage and mycotoxin production in foods.
Problems in maintaining an adequately low aw often occur in the tropics, where high ambient humidity make control of commodity moisture difficult. Where grain is held in bags, systems that employ careful drying and subsequent storage in moisture-proof plastic sheeting may overcome this problem.
While it is possible to control fungal growth in stored commodities by controlled atmospheres or use of preservatives or natural inhibitors, such techniques are almost always more expensive than effective drying, and are thus rarely feasible in developing countries.
7.1.2. Avoid grain damage
Damaged grain is more prone to fungal invasion and therefore mycotoxin contamination. It is thus important to avoid damage before and during drying, and in storage. Drying of maize on the cob, before shelling, is a very good practice.
Insects are a major cause of damage. Field insect pests and some storage species damage grain on the head and promote fungal growth in the moist environment of the ripening grain. In storage, many insect species attack grain, and the moisture that can accumulate from their activities provides ideal conditions for the fungi. To avoid moisture and mould problems, it is essential that numbers of insects in stored grain be kept to a minimum. Such problems are compounded if the grain lacks adequate ventilation, particularly if metal containers are used.
7.1.3. Ensure proper storage conditions
While keeping commodities dry during storage in tropical areas can be difficult, the importance of dry storage cannot be overemphasized. On a small scale, polyethylene bags are effective; on a large scale, safe storage requires well-designed structures with floors and walls impermeable to moisture. Maintenance of the water activity of the stored commodity below 0.7 is crucial.
In tropical areas, outdoor humidities usually fall well below 70% on sunny days. Appropriately timed ventilation, fan-forced if necessary, will greatly assist the maintenance of the commodity at below 0.7 aw. Ideally, all large-scale storage areas should be equipped with instruments for measuring humidity, so that air appropriate for ventilation can be selected.
Sealed storage under modified atmospheres for insect control is also very effective for controlling fungal growth, provided the grain is adequately dried before storage, and provided diurnal temperature fluctuations within the storage are minimised.
If commodities must be stored before adequate drying this should be for only short periods of no more than, say, three days. Use of sealed storage or modified atmospheres will prolong this safe period, but such procedures are relatively expensive and gaslight conditions are essential.
A proven system of storage management is needed, with mycotoxin considerations an integral part of it. A range of decision-support systems is becoming available covering the varying levels of sophistication and scale involved.
7.2. Control
Control of mycotoxin in poultry feed is important and it should be hinged on eliminating mycotoxin from the food chain. Mycotoxygenic fungi are naturally found in soil and air, which makes it difficult to prevent their contamination of agriculture commodities. Nevertheless attempts should be made to control factors that affects the growth of mycotoxigenic fungi and the subsequent toxin production. Factors which include warm temperature between (20oc to 30oc), high moisture content (20-25%), water activity (aw) of about 0.7aw and relative humidity of 70% and above. These factors enhance fungi growth and mycotoxin production (143,145).
Before harvesting of crops damage to grains as a result of field insect pest and some storage species damage grains and promote fungal growth in the environment of ripening grain. Strategies used in various preventive measure in poultry feed involves good agronomic practices, detoxification of mycotoxin in grains use of mould inhibitors, genetic approach through improved breeds of plants.
7.2.1. Physical decontamination
Decontamination of mycotoxin from cereal crops used in the production of poultry feed can be classified as physical decontamination, biological decontamination and chemical decontamination [146]. [145] suggest the following method of elimination of mycotoxin in grains which include, Density segregation and floatation, cleaning and washing, seiving, dehulling, hand picking, irradiation, milling, thermal degeneration.
[147] observed that washing using distill water resulted in 65%-95% reduction of DON (16- 24mg/kg) and 2% to 61% of ZEN (0.9-1.6mg/kg) in contaminated barley and corn.
Density segregation in certain liquid or fractionation by specific gravity help to segregate fungi infected and mycotoxin contamination grains used in the production of poultry feed.[148]. It was also observed that fumonisms present in broken corn kernels is about 10 fold higher than that in intact corn therefore the separation based on the size has been suggested. [147, 146]
Irradiation is also a useful tool in inactivation of some mycotoxin reported ultrasonication been used in contaminated corn without affecting the grain composition. Several workers reported the use of Gamma irradiation to reduce Zearelenone DON and T-2 toxin in corn, wheat. [148-150]
7.2.2. Biological decontamination
This involved systemic degradation of toxins leading to a less toxin product. [151] reports a fungus yeast Expoliata spinnifera was able to grow on fumonisin B1as a sole of carbon source. The hydrolysis of fumonisin B1 yields free Tricarboxylic acid and aminopental, the intermediate aminopentol undergo oxidative deamination. Sacchromyces cerevisiae ferment zearalenone converting it into beta-zearelenol, which has less activity compared to the parent compound.[152]
Feed additive like mycotoxin inactivates trichothecenes by enzymatic decontamination of the 12-13 epoxy ring, and zealenone by the enzymatic opening of the lactone ring [148-152]
7.2.3. Detoxification of mycotoxin feed
Moist ozone and dry ozone were able to reduce DON concentration in contaminated corn up to 90% and 70% respectively [153]. [154] reports 79% reduction in fumonism level in corn.
Author details
Adeniran Lateef Ariyo and Ajagbonna Olatunde Peter
Department of Physiology and Biochemistry, Faculty of Veterinary Medicine, University of Abuja, Nigeria
Sani Nuhu Abdulazeez
Department of Veterinary Pathology, Faculty of Veterinary Medicine, University of Abuja, Nigeria
Olabode Hamza Olatunde
Department of Veterinary Microbiology, Faculty of Veterinary Medicine, University of Abuja, Nigeria
8. References
[1] Simons P. Global production, Consumption and International market of Poultry meat and eggs. World’s Poultry Science Association (WPSA) Poultry Seminar, Lonovala, In September 12 2009
[2] Moss M O. Mycotoxic fungi. In: Microbial Food Poisoning. Elley, A. R. (Ed) Chapman and Hall. London, Glassgow, New York, Tokyo, Melbourne, Madras. 1992 ; Pp 73-106.
[3] D’Mello JPF. Mycotoxins in cereal grains, nuts and other plant products. In: Food Safety Contaminants and Toxins. D’Mello, J P. F.(Ed).Cromwell Press, Trowbridge, UK; 2003. 65-90.
[4] Pitt J I. What are mycotoxins? Mycotoxin News 7:1.1996
[5] Scimeca J A. Naturally occurring orally active dietary carcinogens. In: Handbook of humantoxicology. Massaro, E. J. (Ed), Boka Raton, New York, U.S.A.; 1997. Pp 409-466.
[6] van Egmond HP, Speijers GJA. Survey of data on the incidence and levels of ochratoxin A in food and animal feed worldwide. Natural Toxins 1994; 3: 125-144.
[7] Bhatnagar D, Yu J. Ehrlich K. C. Toxins of filamentous fungi. Chemical Immunology 2002; 81: 167-206.
[8] FA O 1983. Post harvest losses in quality of food grains. Food and Agriculture Organisation (Food and Nutrition Paper No 29; 1983. p. 103.
[9] DeVries JW., Trucksess MW., Jackson LS. Mycotoxins and Food Safety. Kluwer Academic/Plenum Publishers, New York, NY, USA. 2002.
[10] Blount WP. Turkey X disease Turkeys. Journal of the British Turkey Association 1961; 9, 55-58.
[11] Peterson SW, Ito Y, Horn BW, Goto T. Aspergillus bombycis, a new aflatoxigenic species and genetic variation in its sibling species, A. nomius. Mycologia 2001; 93, 689–703.
[12] Ito Y, Peterson SW, Wicklow DT, Goto T. Aspergillus pseudotamarii, a new Aflatoxin producing species in Aspergillus section Flavi. Mycological Research 2001; 105, 233–239.
[13] Frisvad JC, Skouboe P, Samson RA. Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov Systematic Applied Microbiology 2005; 28, 442–453.
[14] Frivad JC, Samson RA, Smedsgaard J. Emericella astellata, a new producer of aflatoxin B1, B2 and sterigmatocystin. Letters in Applied Microbiology 2004; 38, 440–445.
[15] Mircea C, Poiata A, Tuchilus C, Agoroae L, Butnaru E, Stanescu U. Aflatoxigenic fungi isolated from medicinal herbs Toxicology Letters 2008; 180: 32-246.
[16] Makun HA, Dutton MF, Njobeh PB, Gbodi TA, Ogbadu GH. Aflatoxin Contamination in Foods and Feeds: A Special Focus on Africa .Intech; 2012.
[17] D’Mello JPF. Mycotoxins in cereal grains, nuts and other plant products. In: Food Safety Contaminants and Toxins. D’Mello, J P. F.(Ed).Cromwell Press, Trowbridge, UK; 2003. 65-90.
[18] Stössel P. Aflatoxin contamination in soybeans: role of proteinase inhibitors, zinc availability, and seed coat integrity. Applied and Environmental Microbiology 1986; 52, 68–72.
[19] Schindler AF. Temperature limits for production of aflatoxin by twenty-five isolates of Aspergillus flavus and Aspergillus parasiticus. Journal of Food Protection. 1977; 40:39–40.
[20] Ominski KH., Marquardi RR., Sinha RN., Abramson D . Ecological aspects of growth and mycotoxin production by storage fungi. In: Miller, J.D and Trenholm, HL. Mycotoxins in grains: Compounds other than aflatoxins. Eagan Press, St. Paul Minnesota, USA.1994; 287-314.
[21] Codex Alimentarius Commission. Code of Practice for the Prevention and Reduction of Aflatoxin Contamination in Peanuts. 2004. Retrieved August, 2012 from http://www.codexalimentarius.net/download/standards/10084/CXC_055_2004e. pdf.
[22] Kaaya NA., Warren HL. A review of past and present research on aflatoxin in Uganda African Journal of Food Agriculture Nutrition and Development 2005, 18 pages (On Line Free Access)
[23] Agboola S.D 1992. Post harvest technologies to reduce mycotoxin contamination of food crops. In Z.S.C Okoye (ed) Book of proceedings of the first National Workshop on Mycotoxins held at University Jos, on the 29th November, 1990, 73-88.
[24] Anjum MA., Khan SH., Sahota AW, Sardar R. Assessment of Aflatoxin B1 in commercial poultry feed and feed ingredients. The journal of animal and plant sciences 2012; 22, 268-272.
[25] Odoemelam SA., Osu CI. Aflatoxin B1 contamination of some edible grains marketed in Nigeria. E-Journal of Chemistry 2009; 6 (2):308-314.
[26] Adebayo-Tayo BC, Ettah AE. Microbiological quality and aflatoxin B1 level in poultry and livestock feeds. Nigerian Journal of Microbiology, 2010; 24(1): 2145 – 2152.
[27] Kang’ethe EK., Lang.a KA. Aflatoxin B1 and M1 contamination of animal feeds and milk from urban centers in Kenya. African Health Sciences 2009; 9 (4): 218-226
[28] Mngadi PT, Govinden R, Odhav B. Co-occurring mycotoxins in animal feeds. African Journal of Biotechnology 2008; 7 (13): 2239-2243.
[29] Zinedine, A Juan, C Soriano, JM Moltó, JC Idrissi, L and Mañes. J. Limited survey for the occurrence of aflatoxins in cereals and poultry feeds from Rabat, Morocco International Journal of Food Microbiology 2007; 115, 124–127.
[30] Matumba L, Monjerezi M, Khonga EB, Lakudzala DD. Aflatoxins in sorghum, sorghum malt and traditional opaque beer in southern Malawi. Food Control 2011; 22, 266-268
[31] Muthomi JW, Ndung’u JK, Gathumbi JK, Mutitu EW, Wagacha JM. The occurrence of Fusarium species and mycotoxins in Kenyan wheat. Crop Protection 2008; 27, 1215– 1219.
[32] Ghali R, Khlifa KH, Ghorbel H, Maaroufi K, Hedilli A. Incidence of aflatoxins, ochratoxin A and zearalenone in Tunisian foods. Food Control 2008; 19, 921–924.
[33] Riba A., Bouras N., Mokrane S., Mathieu F., Lebrihi A., Sabaou N. Aspergillus section Flavi and aflatoxins in Algerian wheat and derived products. Food and Chemical Toxicology 2010; 48, 2772–2777
[34] Mashinini K, Dutton MF. The incidence of fungi and mycotoxins in South Africa wheat and wheat-based products Journal of Environmental Science and Health Part B, 2006; 41:285-296
[35] Akrobortu DE. Aflatoxin contamination of maize from different storage locations in Ghana. An M.Sc. Thesis submitted to the Department of Agricultural Engineering, Kwame Nkrumah University of Science and Technology, Ghana. 2008; 27-32.
[36] Makun HA, Gbodi TA, Akanya HO, Sakalo AE, Ogbadu HG. Fungi and some mycotoxins contaminating rice (Oryza sativa) in Niger state, Nigeria. African Journal of Biotechnology 2007; 6(2):99–108
[37] Njobeh PB., Dutton MF., Aberg AT., Haggblom P. Estimation of multi-mycotoxin contamination in South African compound feeds. Toxins 2012; 4, 836-848.
[38] Binder EM. managing the risk of mycotoxins in modern feed production Animal Science and Technology. 2007; 133:149-166.
[39] Anjum AD. Outbreak of infectious bursar disease in vaccinated chickens due to aflatoxicosis. Indian Veterinary Journal 1994; 71:322-324.
[40] Asplin FD, Carnaghan RBA. The toxicity of certain groundnut meals for poultry with special reference to their effect on ducklings and chickens. Veterinary Record 1961; 73:1215-1219.
[41] Siller WG, Ostler DC. The histopathology of an entero- hepatic syndrome of turkey poults. Veterinary Record 1961; 73:134-138.
[42] Wannop CC. The bistopathology of turkey "x" disease in Great Britain. Avian Disease 1961; 5:371-381.
[43] Archibald RM, Smith HJ, Smith JD. Brazilian groundnut toxicosis in Canadian broiler chickens. Canadian Veterinary Journal 1962; 3:322-325.
[44] Batra PA, Pruthi K, Sandana IR. Effect of aflatoxin B1 on the efficacy of turkey herpesvirus vaccine against Marek's disease. Reseach in Veterinary Science 1991 ;51:115- 119.
[45] Sharlin 1S, Howarth B, Jr. Wyatt RD. Effect of dietary aflatoxin on reproductive performance of mature White Leghorn males. Poultry Science 1980; 59:1311-1315.
[46] Ortalatli M, Ciftci MK, Tuzcu M, Kaya A. The effects of aflatoxin on the reproductive system of roosters. Research in Veterinary Science 2002; 72:29-36.
[47] Larsen C, Acha M, Ehrich M. Research note: Chlortetracycline and aflatoxin interaction in two lines of chicks. Poultry Science 1988; 67: 1229-1232.
[48] Smith IW, Hill CH, Hamilton PB. The effect of dietary modifications on aflatoxicosis in the broiler chicken. Poultry Science 1971; 50:768-774.
[49] Basic Food Safety for Health Workers.WHO. 1999
[50] Chipley IR, Mabee MS, Applegate KL, Dreyt"us.i MS. Further characterization of tissue distribution and metabolism of (I4C) aflatoxin BI in chickens. Applied Microbiology 1974; 28:1027-1029.
[51] Patterson OSP, Roberts BA. The in vitro reduction of allatoxins Bland 82 by soluble avian liver enzymes. Food Additive and Contaminant 1971; 9:82l-831.
[52] Dutton ME. Fumonisins, Mycotoxins of Increasing Importance:Their Nature and Their Effects. Pharmacology and Therapeutics. 1996; 70( 2) 137-161
[53] Harland EC, Cardeilhac PT. Excretion of carbon14-labeled aflatoxin BI via bile, urine. and intestinal contents of the chicken. American Journal of Veterinary Research 1975; 36:909-912.
[54] Sawhney DS, Vadehra DV, Baker RC. The metabolism of [14C] aflatoxins in laying hens. Poultry Science 1973; 52:1302-1309.
[55] Oliveira CA, Kobashigawa E, Reis TA, Mestieri L, Albuquerque R, Correa B. Aflatoxin B I residues in eggs of laying hens fed a diet comaining different levels of the mycotoxin. Food Additive and Contaminant 2000; 17:459-462.
[56] Jacobson WC, Wiseman HG. The transmission of aflatoxin BI into eggs. Poultry Sciience 1974; 53:1743-1745.
[57] Trucksess MW, Stoloff L, Young K, Wyatt RD, Miller BL. Aflatoxicol and aflatoxins B I and MI in eggs and tissues of laying hens consuming aflatoxin-contaminated feed. Poultry Science 1983; 62:2176--2182.
[58] Sova Z, Fukal L, Trefuy D, Prosek I, Slamova A. Bl aflatoxin (AFBI) transfer from reproductive organs of farm birds into their eggs and hatched young. Conference of European Agriculture 1986;7:602-603.
[59] Lillehoj EB, Aalund O, Hald B. Bioproduction of 14C Ochratoxin A in submerged culture, Applied Environmental Microbiology. 1978; 36 : 720–723..
[60] Foster PL, Eisenstadt E, Miller JH. Base substitution mutations induced by metabolically activated aflatoxin B1. Proceeding of National Academic of Science 1983; 80 : 2695–2698.
[61] Raney KD, Meyer DJ, Ketterer B, Harris TM, Guengerich FP. Glutathione conjugation of aflatoxin B-1 exo- and endo-epoxides by rat and human glutathione-S-transferases. Chemical Research Toxicology 1992; 5 :470–478.
[62] Bababunmi EA, Thabrew I, Bassir O. Aflatoxin induced coagulopathy in different nutritionally classified animal species. World Review of Nutrition and Diet 1997; 34 : 161–181.
[63] Shen M, Ong CN, Shi CY. Involvement of reactive oxygen species in aflatoxin B1- induced cell injury in cultured rat hepatocytes. Toxicology 1997; 99 :115–123
[64] Bonsi GP, Agusti-Tocco M, Palmery M, Giorgi M. Aflatoxin B1 is an inhibitor of cyclic nucleotide phosphodiesterase activity. General Pharmacoogy 1991; 32: 615–619.
[65] Dwivedi P, Burns RB. The natural occurrence of ochratoxin A and its effects in poultry. A review. Part I. Epidemiology and toxicity. World Poultry Science Journal 1986; 42:32- 47.
[66] Rosa C A R, Ribeiro J M M, Fraga M J, Gatti M, Cavaglieri L R, Magnoli C E, Dalcero A M, Lopez C WG. Mycoflora ofpoultry feeds and ochratoxin-producing ability of isolated Aspergillus and Penicillium species. Veterinary Microbiology 2006; 113:89-96.
[67] Rizzo I, Vedoya G, Maurotto S, Haidukowski M, Varkavsky E. Assessment of oxinogenic fungi on Argentinean medicinal herbs, Microbiology Research 2004; 159:113–120.
[68] Sawhney DS, Vadehra DV, Baker RC. The metabolism of [14C] aflatoxins in laying hens. Poultry Science 1973; 52:1302-1309.
[69] Larsen TO, Svendsen A, Smedsgaard J. Biochemical characterization of ochratoxin Aroducing strains of the genus Penicillium, Applied Environmental Microbiology 2001; 67: 3630–3635.
[70] Castella G, Larsen TO, Cabanes J, Schmidt H, Alboresi A, Niessen L, Geisen R. Molecular characterization of ochratoxin A producing strains of the genum Penicillium, Systemic and Applied Microbiology 2002; 25 : 74–83
[71] Kozakiewicz Z. Aspergillus species on stored products, Mycological paper no. 161, CAB International Mycological Institute, Kew, UK, 1989, p. 1.
[72] Pardo E, Mar´in S, Ramos AJ, Sanchis V. Effect of water activity and temperature on mycelial growth and ochratoxin A production by isolates of Aspergillus ochraceus onirradiated green coffee beans, Journal of Food Protection 2005; 68 : 133–138.
[73] Basic Food Safety for Health Workers.WHO. 1999
[74] Abarca M, Bragualt M R, Castella G, Cabanes F J. Ochratoxin A production by strains of Aspergillus niger var niger, Applied Environmental Microbiology 1994; 60: 2650–2652.
[75] Belli N, Marin S, Sanchis V, Ramos AJ. Influence of water activity and temperature on growth of isolates of Aspergillus section nigri obtained from grapes, International Journal Food Microbiology 2004; 96 : 19–27.
[76] Teren J, Varga J, Hamari Z. Immunochemical detection of Ochratoxin A in black Aspergillus strain, Mycopathologia 1996;134 :171–176.
[77] Mitchell D, Parra R, Aldred D, Magan N. Water and temperature relations of growth and ochratoxin A production by Aspergillus carbonarius strains from grapes in Europe and Israel, Journal Applied Microbiology 2004; 97: 439–445.
[78] Rosa C A R, Ribeiro J M M, Fraga M J, Gatti M, Cavaglieri L R, Magnoli C E, Dalcero A M, Lopez C WG. Mycoflora Of poultry feeds and ochratoxin-producing ability of isolated Aspergillus and Penicillium species. Veterinary Microbiology 2006; 113:89-96.
[79] Accensi F, Abarca M.L, Cabanes FJ. Occurrence of Aspergillus species in Mixed feeds and component raw materials and their ability to produce ochratoxin A, Food Microbiology 2004; 21 : 623–627.
[80] Beg MU, Al-Mutairi M, Beg KR, Al-Mazeedi HM, Ali LN, Saeed T, 2006. Mycotoxins in poultry feed in Kuwait. Archive of Environmental Contaminant. Toxicology 2006; 50, 594–602.
[81] Cabanes FJ, Accensi F, Bragualt MR, Abarca ML, Minguez G, Pons S. What is the source of ochratoxin A in wine? International Journal Food Microbiology 2002;79 : 213–215.
[82] Samson RA, Houbraken JAMP, Kujipers AFA, Frank JM, Frisvard, JC. New ochratoxin A or sclerotium producing species in Aspergillus section Nigri, Study.Mycology 2004;50 : 45–61.
[83] Moss MO. Mode of formation of ochratoxin A, Food Additive and Contaminant. 1996;13 : 5–9.
[84] ayman P, Baker JL, Doster MA, Michailidis TJ, Mahoney NE. (2002).Ochratoxin production by the Aspergillus ochraceus group and Aspergillus alliaceus, Applied Environmental Microbiology 2002; 68 : 2326–2329.
[85] Varga J, Rigo K, Toth B, Teren J, Kozakiewicz Z. Evolutionary relationships among Aspergillus species producing economically important mycotoxins, Food Technology and Biotechnology 2003; 41 : 29–36.
[86] Callaghan JO, Caddick MX, Dobson AD. A polyketide synthase gene required for ochratoxin A biosynthesis in Aspergillus ochraceus, Microbiology 2003;149 : 3485–3491.
[87] Beg MU., Al-Mutairi M., Beg KR., Al-Mazeedi HM., Ali LN., Saeed T. Mycotoxins in poultry feed in Kuwait. Archive of Environmental Contaminant & Toxicology. 2006;50, 594–602
[88] Figueroa S, Centeno, S, Calvo MA, Rengel A, Adelantado C. Mycobiodata and concentration of ochratoxin A in concentrated poultry feed from Venezuela. Pakistan Journal of Biological Science 2009; 12: 589–594
[89] Dalcero A, Magnoli C, Hallak C, Chiacchiera SM, Palacio G, Rosa CAR. Detection of ochratoxin A in animal feeds and capacity to produce this mycotoxin for Aspergillus section Nigri in Argentina. Food Additives and Contaminants. 2002; 19 (11), 1065–1072.
[90] Zafar, F., Yasmin, N., Hassan, R., Naim, T., Qureshi, A.A. A study on the analysis of ochratoxin A in different poultry feed ingredients. Pakistan Journal of Pharmaceutical Sciences 2001;14, 5–7.
[91] Duarte SC, Lino CL, Pena A. Ochratoxin A in feed of food-producing animals: An undesirable mycotoxin with health and performance effects. Veterinary Microbiology 2011; 154, 1–13.
[92] Bozzo G., Bonerba E, Ceci E, Colao V, Tantillo G. Determination of ochratoxin A in eggs and target tissues of experimentally drugged hens using HPLC–FLD. Food Chemistry 2011; 126, 1278–1282.
[93] Sakthivelan SM, Rao GVS. Effect of ochratoxin A on body weight, feed intake and feed conversion in broiler chicken. Veterinary Medical Int. 2010; 1–4.
[94] Bozzo G, Ceci E, Bonerba E, Desantis S, Tantillo G. Ochratoxin A in laying hens: highperformance liquid chromatography detection and cytological and histological analysis of target tissues. Journal of Applied Poultry Research 2008; 17, 151–156.
[95] Elaroussi MA, Mohamed FR, Barkouky EME, Atta AM, Abdou AM, Hatab MH. Experimental ochratoxicosis in broiler chickens. Avian Pathology 2006; 35, 263–269.
[96] International Agency for Research on Cancer. 1993. Toxins derived from Fusarium moniliforme: fumonisins B1 and B2 and Fusarium C, p. 445-466. In IARC Monographs on the evaluation of the carcinogenic risks to humans: some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins, vol. 56. International Agency for Research on Cancer, Lyon, France.
[97] Marasas WFO. Fumonisins: history, worldwide occurrence and impact, p. 1-17. In L. S. Jackson, J. W. DeVries, and L. B. Bullerman (ed.), Fumonisins in food. Plenum Press, New York, N.Y; 1996.
[98] Branham, B. E., and R. D. Plattner. 1993. Alanine is a precursor in the biosynthesis of fumonisin B1 by Fusarium moniliforme. Mycopathologia 124:99-1
[99] Marín S, Sanchis V, Magan N. Water activity, temperature, and pH effects on growth of Fusarium moniliforme and Fusarium proliferatum isolates from maize. Canadian Journal of Microbiology 1995a; 41:1063-1070.
[100] Marín S, Sanchis, V, Vinas I, Canela R, Magan N. Effect of water activity and temperature on growth and fumonisin B1 and B2 production by Fusarium proliferatum and F. moniliforme in grain. Letter of Applied Microbiology 1995b; 21:298-301.
[101] Sydenham EW, Shephard G S, Thiel P G, Marasas, W E, Rheeder J I, Peralta C E, Gonzalez H L, Resnik S L. Fumonisins in Argentinian field trial corn. Journal of Agriculture and Food Chemistry 1993; 41: 891-895.
[102] Doko M B, Rapior S, Visconti A, Schjoth J E. Incidence and levels of fumonisin contamination in maize enotypes grown in Europe and Africa. Journal of Agriculture and Food Chemistry 1995. 43: 429-434.
[103] Sydenham E W, Shephard G S, Thiel PG, Marasas W F 0, Stockenstrom S. Fumonisin contamination of ommercial corn-based human foodstuffs. Journal of Agriculture and Food Chemistry 1991; 39: 2014-2018.
[104] Ueno Y, Aoyama S, Suglaru Y, Wang D S, Lee U S, Hirooka EY, Ham S, Karki T, Chcn G, Yu S Z. A limited survey of fumonisins in corn and corn-based products in Asia countries. Mycotoxin. Research 1993; 9: 27- 34
[105] Julian A M, Wareing IW, Phillips SI, Medlock V F, MacDonald M V, de1 Rio L E. Fungal contamination and selected mycotoxins in pre and postharvest maize in Honduras. Mycopathologia 1995; 129: 5-16.
[106] Doko MB., Canet C., Brown N., Sydenham EW., Mpuchane S., Siame B A. Natural cooccurrence of fumonisins and zearalenone in cereals and cereal-based foods from eastern and southern Africa. Journal of Agriculture and Food Chemistry.1996; 44, 3240- 3243.
[107] Julian A M, Wareing IW, Phillips SI, Medlock V F, MacDonald M V, de1 Rio L E. Fungal contamination and selected mycotoxins in pre and postharvest maize in Honduras. Mycopathologia 1995; 129: 5-16.
[108] Pineiro MS., Silva GE., Scott PM., Lawrence GA., Stack ME. Fumonisin level in Uruguayan corn products. Journal of AOAC International 1997; 80, 825-828
. [109] Viquez OM., Castell-Pere M.E., Shelby RA. Occurrence of fumonisin B1 in maize grown in Costa Rica. Journal of Agriculture and Food Chemistry. 1996; 44, 2789-2791.
[110] Prathapkumar S H, RaoVS, Paramkishanand UR, Bha RV. Disease outbreak in laying hens arising from the consumption of fumonisin-oontaminated food. Br Poultry Science 1997; 38:475-479.
[111] Chatterjee D S, MukheJjee K, Dey A. Nuclear disintegration in chicken peritoneal macrophages exposed to fumonisin BI from Indian maize. Applied Microbiology 1995; 20:184-185.
[112] Dombrink-Kurtzman MA, Javed T, Bennett G A, Richard J L, Cow L, Buck W B. Lymphocyte cytotoxicity and erythrocytic abnormalities induced in broiler chicks by fumonisins B, and B1 and moniliformin from Fusarium proliferatum. Mycopathologia 1993; 124: 47-54.
[113] Javed I, Bennett G A, Richard J L, Dombrink Kurtzman MA, Cote L M, Buck W B. Mortality in broiler chicks on feed amended with Fusarium proliferatum culture materials or with purified fumonisin B and moniliformin. Mycopathologia; 1993; 123: 171-184.
[114] Marasas,VI E. Mycotoxicological investigations on corn produced in esophageal cancer areas in Transkei. In: Cancer of the Esophagus, 1982 Vol. 1, pp. 29-40, Pfeiffer, C J (ed.) CRC Press Inc, Boca Raton.
[115] Vesonder R, Halihurton J, Golinski P. Toxicity of field samples and Fusarium moniliforme from feed associated with equine- leucoencephalomalacia Archive of Environmental Contaminant 1989; 18: 439-442.
[116] Adejumo TO, Hettwer U, Karlovsky P. Occurrence of Fusarium species and trichothecenes in Nigerian maize. International Journal of Food Microbiology. 2007; 116: 350–357
[117] Dutton MF., Kinsey A. A note on the occurrence of mycotoxins in cereals and animal feedstuffs in Kwazulu Natal, South Africa 1984-1993. South Africa Journal of Animal Science. 1996; 26, 53-57.
[118] Pacin AM., Resnik SL., Neira MS., Molto G., Martinez E. Natural occurrence of deoxynivalenol in wheat, wheat flour and bakery products in Argentina. Food Additive and Contaminant. 1997; 14, 327-331.
[119] Furlong EB., Soares LMV., Lasca CC., Kohara EY., 1995. Mycotoxins and fungi in wheat harvested during in test plots in the state of Sao Paulo Brazil. Mycopathologia 1990; 131, 185-190.
[120] Salma K, Nafeesa Q H, Tahira I, Sultana N, Sultana K, and Ayub N. Natural occurrence of aflatoxins, zearalenone and Trichothecenes in maize grown in Pakistan, Pakistan Journal of Botanical 2012; 44(1): 231-236
[121] Perkowski J., Plattner RD., Golinski P., Vesonder RF. Natural occurrence of deoxynivalenol, 3-acetyldeoxynivalenol, 15-acetyl-deoxynivalenol, nivalenol, 4,7- dideoxynivalenol and zearalenone in Polish wheat. Mycotoxin Research. 1990; 6, 7-12.
[122] Vrabcheva T., Gebler R., Usleber E., Martlbauer E. First survey on the natural occurrence of Fusarium mycotoxins in Bulgarian wheat. Mycopathologia. 1996; 136, 47- 52.
[123] Hietaniemi V., Kumpulainen J. Contents of Fusarium toxins in Finnish and imported grains and feeds. Food Additive and Contaminants. 1991; 8, 171-182.
[124] Langseth W., Elen O. Differences between barley, oats and wheat in the occurrence of deoxynivalenol and other trichothecenes in Norwegian grain. J. Phytopathology. 1996; 144, 113-118.
[125] Tanaka T., Yamamoto S., Hasegawa A., Aoki N., Besling JR., Sugiura, Y., Ueno Y. A survey of the natural occurrence of Fusarium mycotoxins, deoxynivalenol, nivalenol and zearalenone, in cereals harvested in The Netherlands. Mycopathologia. 1990; 110, 19-22.
[126] Rheeder JP., Sydenham EW., Marasas WFO., Thiel PG., Shephard GS., Schlechter M., Stockenstrom S. .. Viljoen JH. Fungal infestation and mycotoxin contamination of South African commercial maize harvested in 1989 and 1990. South Africa Journal of Science.1995; 91, 127-131.
[127] Yamashita A., Yoshizawa T., Aiura Y., Sanchez PC., Dizon EI., Arim, RH., Sardjono, , 1995. Fusarium mycotoxins (fumonisins, nivalenol and zearalenone) and aflatoxins in corn from southeast Asia. Biological science.Biotechnology. Biochemistry. 1995; 59, 1804-1807.
[128] Ryu JC., Yang JS., Song YS., Kwon OS., Park J., Chang IM. 1996. Survey of natural occurrence of trichothecene mycotoxins and zearalenone in Korean cereals harvested in 1992 using gas chromatography/ mass spectrometry. Food Additive and Contamination. 1996; 13, 333-341.
[129] Wang DS., Liang YX., Iijima K., Sugiura Y., Tanaka T., Chen G., Yu SZ., Ueno Y. Cocontamination of mycotoxins in corn harvested in Haimen, a high risk area of primary liver cancer in China. Mycotoxins 1995b; 41, 67-70.
[130] Yoshizawa T. Geographic difference in trichothecene occurrence in Japanese wheat and barley. Bull. Institute of. Comprehensive Agricultural Science. Kinki University 1997; 5, 23-30.
[131] Lauren DR., Jensen DJ., Smith WA., Dow BW., Sayer ST. Mycotoxins in New Zealand maize: a study of some factors influencing contamination levels in grain New Zealand. Journal of Crop and Horticultural Science. 1996; 24, 13-20.
[132] Fernandez C., Stack ME., Musser SM. Determination of deoxynivalenol in 1991 U.S. winter and spring wheat by high-performance thin-layer chromatography. Journal of AOAC Institute.1994; 77, 628-630.
[133] Stratton GW., Robinson AR., Smith HC., Kittilsen L., Barbour M. Levels of five mycotoxins in grains harvested in Atlantic Canada as measured by high performance liquid chromatography. Architecture of Environmental Contamination and Toxicology.1993; 24, 399-409.
[134] Scott PM. Multi-year monitoring of Canadian grains and grain-based foods for treichothecenes and zearalenone. Food Additive and Contaminant. 14, 333-339.
[135]
[136] Diaz G.J., Squires E. J., Julian R. J. And Boermans H.J. Individual and combined effects of T-2 toxin and DAS in laying hens and Broiler Poultry Science 1994; 35; 393 - 405
[137] Brake J, Hamilton PB, Kittrell R S. Effects of the Trichothecene Mycotoxin Diacetoxyscirpenol on Feed Consumption, Body Weight, and Oral Lesions of Broiler Breeders. Poultry Science, 2000; 79:856–863
[138] Ademoyero A. A, Hamilton PB. Influence of degree of acetylation of scirpenol mycotoxins on feed refusal by chickens. Poultry Science 1989; 68:854–856.
[139] Jakic-Dimic D, Nešic K, Šefer D. Mycotoxicoses of poultry caused by Trichothecenes. Biotechnology in Animal Husbandry 2011; 27 (3), p 713-719. DOI: 10.2298/BAH1103713J
[140] Rafai P, Pettersson H, Bata A, Papa Z, Glavits S, Tubolys S,Vanyi A, Sooss P. Effect of dietary T-2 fusariotoxin concentrations on the health and production of white Pekin duck broilers. Poultry Science 2000; 79, 1548-1556.
[141] Scott ML., Dean WF. In: Nutrition and Management of Ducks. M.&. Scott, Ithaca, NY; 1991. 150-166.
[142] Vanyi A, Bata A, Kovacs F. Effects of T-2 toxin treatment on the egg yield and hatchability in geese. Acta Veterinaria Hungarica 1994; 42, 1, 79-85.
[143] Langseth W., Hqie R. Gullord M. The influence of cultivars, location and climate on deoxynivalenol contamination in Norwegian oats (1985-1990). Acta Agriculture Scandinavica. Section B: Soil and Plant Science, 1995 45: 63-67.
[144] Ma H., Zhon M., Liu Z. Liu W. Progress on genetic improvement for resistance to wheat scab in KLA. Journal of Applied Genetics, 2002; 43: 259-266.
[145] Negedu A., Atawodi SE. Ameh JB., Umoh VJ., Tanko HY. Economic and health perspectives of mycotoxins: a review. Continental Journal of Biomedical Sciences 2011; 5 (1): 5 – 26.
[146] Gbodi TA., Makun HA., Kabiru YA., Ogbadoyi E., Tijani SA., Lawal SA., Bayero RA. Effect of local processing methods on aflatoxin contents of some common Nigerian foods prepared from artificially contaminated maize, rice and sorghum. Journal of Agricultural Science. Mansoura University. 2001; 26: 3759 -3769.
[147] Desjardins AE., Manandhar GG., Plattner RD., Maragos CM., Shrestha K.. McCormick SP. (2000). Occurrence of Fusarium species and mycotoxins in Nepalese maize and wheat and the effect of traditional processing methods on mycotoxin levels. Journal of Agricultural food Chemistry. 2000; 48:1377 – 1383.
[148] Scott PM. Mycotoxins transmitted into beer from contaminated grains during brewing. Journal of the Association of Official Analytical Chemists International. 1996; 79:675.
[149] Diaz DE., Hopkins BA., Leonard LM. Hagler WM. and Whitlow LW. Effects of fumonisin on lactating dairy cattle. J ournal Dairly Science. 2000. 83 (Abstract): 1171.
[150] Fanelli C., Taddei F., Jestoi M. and Visconti A. Use of resvertrol and BHA to control fungal growth and mycotoxin production in wheat and maize seeds. Aspect of Applied Biology. 2003. 68: 63-71.
[151] Duvick J., Rood TA., Maddox JR., and Gilliam J. Advances in ochratoxin A biosynthesis. Book of Abstracts, International Conference on “Advances on genomics, biodiversity and rapid systems for detection of toxigenic fungi and mycotooxins” September 26-29, 2006. Monopoli (Bari), Italy Pp.39 Retrieved from http://www.Ispa.onr.n/mycoglobe-2006. 1998.
[152] Leggot NL and Shephard G. Patulin in South African commercial apple products. Food Control. 2001. 12 (2) 73-76
[153] Diaz DE., and Smith TK. Mycotoxin sequestering agents: Practical tools for the neutralization of mycotoxins in the mycotoxin Blue Book. D. Diaz, ed. Nottingham Univ. Press, Nottingham, U. K. 2005.Pp. 313-339
[154] Park JW., Scott PM., Lau BPY., and Levis D A. Analysis of heat processed corn foods for fumonisins and bound fumonisns. Food Additives and Contaminants. 2004; 21:1168- 1178.
This chapter 4 is part of "Mycotoxin and Food Safety in Developing Countries", book edited by Hussaini Anthony Makun, ISBN 978-953-51-1096-5, Published: April 10, 2013 under CC BY 3.0 license
Related topics
Authors:
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a postBiotek Indonesia
1 de octubre de 2014
Dear Sir, Why dont you have information about Avian Mybotoxicosis in Indonesia?









.jpg&w=3840&q=75)