Uses and Abuses of NIR for Feed Analysis
Published: July 17, 2013
By: Dan Undersander (University of Wisconsin)
Introduction
Dairymen use feed and forage testing to reduce feed costs and maximize production. As such, accuracy of the feed tests are of great concern. The true objective of a forage or feed analysis is to predict animal performance from the forage and feed used in a ration formulated to meet nutrient requirements. This means that dairymen must consider, first, does the test relate to animal performance and, second, what the error of analysis is. In this regard many dairymen and nutritionists have debated the value of near infrared reflectance spectroscopy (NIR or NIRS). This paper will describe NIR, present some data on use of NIR, good and bad, and some potentials NIR presents.
What is NIR?
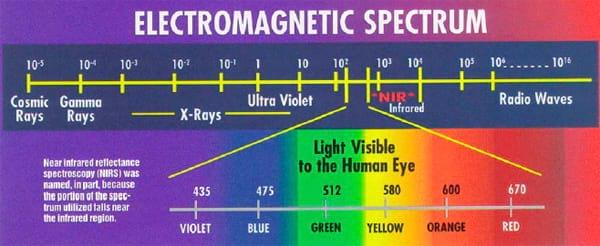
Light is described in wavelengths as shown in the graph below. Visible light is basically light with wavelengths from 400 to 700 nanometers (nm). (A nanometer is 1 millionth of a meter or 0.000039 inches.) Infrared light is that light which has wavelengths just longer than the visible range. It occurs naturally from the sun and causes tillering and reproductive changes in plants. While we cannot see infrared, we can feel the heat it causes when absorbed.
When we 'see' something, we see the reflected light. So, if something appears blue, it is because that item reflects blue light and absorbs the other visible light wavelengths. Unlike visible light, reflected infrared light to the composition of an item, especially the hydrogen bonds. If we could see infrared light, we would not see color but rather 'protein,' 'starch,' 'fat,' etc.
Instruments are available that can detect near infrared light reflectance. These NIR instruments, if properly calibrated, can detect infrared light and interpret the kind and amount of organic compounds present. NIR is a rapid, nondestructive method of analysis requiring minimal sample preparation that was first used to predict forage quality in 1976. NIR has been approved by the Association of Official Analytical Chemists (AOAC) for use in determining moisture, Kjeldahl nitrogen and acid detergent fiber for feed and forage analysis. It is used by grain terminals across the U.S. and around the world for determining moisture and protein in grain.
Proper Use of NIR
First we must recognize that an NIR is not a black box. It is a tool, like any other, that can produce good results if used properly and poor results if improperly used. All tools, like scales, gas chromatographs and others, will produce good or bad results depending on the operator.
Next we must recognize that NIR can be no better than the wet chemistry used to calibrate the instrument. If bad calibration values are used, then the NIR will predict incorrectly. Forage laboratory error comes from two factors 1) sub sampling of the forage sample taken and 2) poor analysis procedure.
The dairyman can take two actions to help reduce laboratory error due to improper sub sampling. The first is to sample correctly in the field (using cores from bales or hand samples from silos) and then to mix thoroughly and sub sample so that less than 1 lb forage is sent in to the laboratory. The second action is for the dairyman to ask the laboratory how samples are handled and to discourage further subsampling once the sample has arrived at the laboratory.
The next question the dairyman should be concerned about is how well the laboratory is performing forage analysis. The National Forage Testing Association sends out check samples to laboratories six times annually and reports back to the laboratories on how they are doing. Laboratory performance is based on analysis of four parameters (DM, CP, ADF, and NDF). A summary of these results for the approximately 136 participating laboratories is presented in Table 1. Dry matter was not included because the DM reported is laboratory DM, not original sample DM.
Table 1. Performance of forage testing laboratories participating in National Forage Testing Association check sample program, 2004
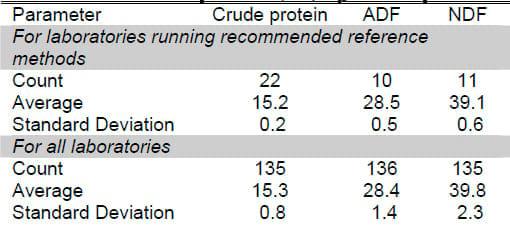
The first thing to note is that few laboratories are using recommend reference methods. In fact, only 10 or 11 laboratories out of 136 are using the recommended method for ADF and NDF! Recommended procedures are based on methods that produce consistently small error between repeated tests and accuracy of the value needed. It is easy to understand why different laboratories are getting different results when they are using different methods.
The second important consideration is that laboratories using the reference methods had low standard errors. Standard deviations were 0.2, 0.5 and 0.6 for CP, ADF and NDF, respectively. A standard deviation is the range that includes repetitions 66% of the time. So, in the above table, we would expect repeats of crude protein analysis among laboratories using recommended reference methods to be within 0.2% two-thirds of the time. The table shows that laboratories can get good, repeatable analyses on the same sample when recommended methods and good laboratory procedures are used.
The second portion of the table gives the error for laboratories participating in check sample programs but not using recommended methods. Errors are 0.8, 1.4 and 2.3 % for CP, ADF and NDF, respectively. Therefore, we would expect repeats of crude protein analysis among laboratories generally to be within 0.8% two-thirds of the time. In other words, laboratory to laboratory variation is such that repeats among laboratories would be expected to exceed those numbers 34% of the time. So dairymen should make sure the forage testing laboratory they use is participating in the NFTA national proficiency testing program and ask for results to make sure that the laboratory analytical accuracy meets your expectations.
This whole process becomes more pronounced as we move into new measures such as in vitro digestibility and RUP. While these tests are likely to more accurately predict animal performance, there are no nationally agreed upon procedures. So there is no reason to expect different laboratories to produce similar results. Further within laboratory variability is likely to be high. Remember, NIR can be no better than the wet chemistry it represents!
If a laboratory has good wet chemistry, the next question is whether or not the NIR instrument is being properly operated. As with a scale or other measuring device, the instrument must be monitored to make sure that it is operating properly. There are procedures and tools for doing this. Some laboratories do this routinely, some do not.
The next step is to ask if the calibration equations of the laboratory are appropriate for the samples being analyzed - does the NIR equation include samples of the type to be analyzed, grown in the region of the sample to be analyzed, does it include drought stress samples, etc. Equations cannot accurately predict composition if they contain no samples of the type being analyzed. A good laboratory should recognize the limitations of their equations and use wet chemistry where a sample falls outside the range of samples included in equations.
Misuse of NIR
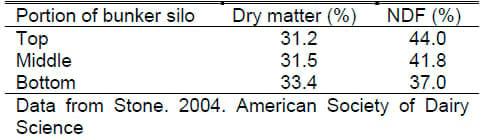
The biggest misuse of NIR is to start with a bad sample. There is significant variation among lots of grain, byproducts, hay or silage. Note the variation in different portions of a bunker silo as reported by Stone. Those taking a sample for analysis must be carefully trained and standard sampling protocols rigidly adhered to. Recommended procedures for sampling are available on the National Forage Testing Association (NFTA) website at: http://www.foragetesting.org/lab_procedure/appendixe.html and the University of Wisconsin Extension Service at: http://cecommerce.uwex.edu/pdfs/A2309.PDF.
Table 2. Variation in Corn Silage Composition in Bunker Silo
A common mistake is to assume that one wet chemistry analysis (at higher cost) is always better than NIR when multiple NIR sample analyses would frequently provide a better answer at the same or lower cost. The largely unused opportunity with NIR is to take and analyze multiple samples. The lower cost of NIR will make this cost effective. Running multiple samples will greatly reduce the error of analysis (see below).
Another common mistake is so assume that NIR is always wrong when the NIR and a wet chemistry analysis result differ. We have been working diligently to develop good NIR equations in a joint industry-university-USDA-ARS effort through the NIRS Consortium. These equations are to the point that, when a wet chemistry protein is run and differs from the Consortium equation, a rerun of the wet chemistry will agree with the NIR about 80% of the time. The wet chemistry reruns for ADF and NDF analyses agree with the NIR about 50% of the time. In short, if you have one NIR and one wet chemistry analysis that differ, you have no way of knowing which is correct!
A common misuse of NIR is for a laboratory to report NIR analyses for a sample type not in the equation. This happens commonly for different byproduct feeds which may vary widely in sample type and not be analyzed too frequently. It also commonly happens for forages where a load of hay, for example, is of a type not commonly analyzed by the laboratory or is bought outside the region where samples usually come from. It is common, especially for large dairies to buy feed and forage from widely differing regions. For this reason the NIRS Consortium has made a concerted effort to develop equations that are truly national in scope and cover as many different environments as possible.
Another common mistake is to assume that the sample dry matter determination on the NIR analysis report has been determined by NIR. This is almost never the case if dry matter is less than 85%. Wet chemistry dry matter is the most variable analyte, both among and within laboratories. NIR can determine moisture very well (and is more accurate than most wet chemistry analyses) but most laboratories are not equipped to read samples wetter than about 15% moisture. Therefore they partially dry the sample (either in a microwave or a convection oven) and then grind and scan the sample when it is less than 15% moisture. The final dry matter is determined as the weight lost during the initial drying and the residual moisture read by the NIR.
Potential Uses of NIR
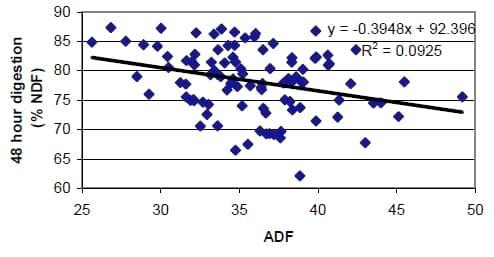
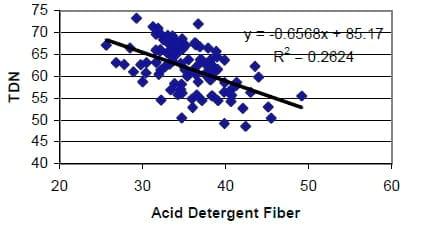
New Forage Tests: Forage tests must not only be repeatable (precise) and run correctly (accurate) but this must predict something important to the animal to be useful when balancing rations. We have long used acid detergent fiber (ADF) to estimate energy content of forages. The assumptions are that all fiber digests the same and that increased fiber means less energy. Both assumptions are wrong and the relationship between ADF and energy content of the forage, while related, are not close enough for modern ration balancing (Figure 2). This means that even though the ADF analysis may be performed accurately, it may not relate to animal performance as much as assumed.
Figure 2. Comparison of 48-Hr in vitro digestion of ADF of random samples submitted to forage testing laboratory
This is why the NRC 2001 Requirements for Dairy Animals recommend using TDN to estimate energy rather than previous procedures (Figure 3). The reason we haven't done this all along is that several analyses are needed and they were too expensive and slow to run by wet chemistry. A key component of this analysis is to run digestible fiber. Clearly digestible fiber varies and should be analyzed as shown in figure 4. In fact, nutritionists who are using digestible fiber, report great improvements in accuracy of ration balancing for dairy cows.
Figure 3. Comparison of acid detergent fiber to TDN (NRC 2001) of random samples submitted to forage testing laboratory.
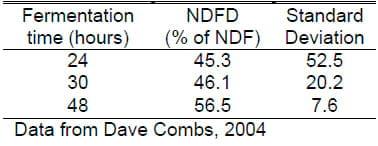
One issue that has arisen is the time of in vitro digestion for fiber. It is well known that forage passes through the animal faster and less is digested as level of feed intake increases. NRC recommends 48-hour digestion, which is approximately equal to the rumen retention time of forage in a cow at maintenance. This value is then adjusted downward as level of feed intake is increased. Some have recommended shorter in vitro incubation times of 24 or 30 hours to more closely reflect the time the forage actually stays in the rumen. The two problems with shorter rumen retention times are they reflect average rumen retention time, not the time that individual particles stay in the rumen and, more importantly, error of estimate increases dramatically as in vitro fermentation time is decreased. This is dramatically shown in Table 3 where going from 48 hour digestion to 24 hour digestion increased the standard deviation from 7 to 52! Thus the error was greater than the mean in this trial for 24 hour in vitro estimates. Further studies from several laboratories have shown that most forages rank about the same over all time periods of digestion. The error comes, in large part, from greater impact of the lag time as the fermentation time is shorter. NIR might be able to improve on this over wet chemistry because great replication of calibration standards could reduce the run-to-run error and increase predictability of shorter fermentation times over wet chemistry in vitro or in situ determinations.
Table 3. Effect of rumen fermentation time on NDF digestibility and errors of measurement
We have actually developed digestion kinetic equations for forages. This is an example of NIR estimates that could be come available to dairymen which are too expensive and time consuming to determine by in vitro or in situ digestion for individual samples.
Another potential is that NIR can be used to provide in vivo estimates of energy (Berglund et al. 2000). In Northern Ireland, NIR equations have been developed to predict in vivo digestion and intake of grass silages. Samples are analyzed fresh (undried and unground) and results reported to farmers.
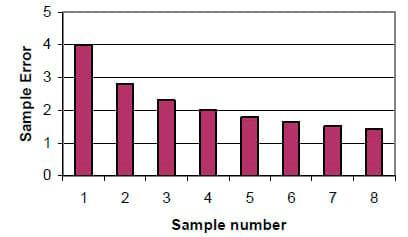
Reducing error through multiple sampling and analysis: One of the practical ways to get the most accurate forage analysis that most nutritionists have missed is to run multiple samples. As shown in Figure 3, errors of estimate decrease dramatically as sample numbers increase. This means that the accuracy is increasing. Thus rather than accepting samples results with high errors because of sampling error, laboratory error of the technique, etc, the dairymen/nutritionist should submit multiple samples. Reducing the error submitting four samples rather than one will reduce the error from 4 to 2 when results are averaged to give better estimates of the composition of the feedstuff. This can be cost effective. Some dairymen have asked for wet chemistry analysis, mistaken assuming that the errors will be greatly minimized compared to near infrared analysis (NIR). While the errors are somewhat higher for NIR, sample errors and error of techniques still exist. The big advantages of NIR are that it is much less expensive and more repeatable. Therefore a farmer would get better results by running multiple samples by NIR than a single sample by wet chemistry when the cost would be the same or less.
In summary, Near Infrared Reflectance Spectroscopy (NIR) can accurately predict composition of feedstuff. It is widely used around the world. NIR can improve forage analysis through rapid, inexpensive analysis that allows multiple samples to be run, improving analysis accuracy.
Figure 3. Effect of multiple samples on sampling error
Literature Cited
Berglund, I., K. Larsson and W. Lindberg. 1990. Estimation of metabolisable energy for ruminants by near infrared reflectance photometry using multivariate methods. J. Sci. Food Agric. Essex: Elsevier Applied Science. v. 52 (3) p. 339-349
NRC, 2001. Nutrient Requirements of Dairy Cattle, Seventh Revised Edition. National Research Council, National Academy Press, Washington, DC.
Related topics
Authors:
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a postUniversity of Wisconsin - USA
23 de septiembre de 2013
Phytate can be measured if a good data base of samples with chemistry exists to build the initial NIR Equation. The reliability and difference among equations for feed types depends on how the NIR equation is build. A reliable equation could be build to work across grain types.
Many farmers have not bought real time NIR devices because of the cost and the extra time involved. I think it will eventually become more common.
IFF - International Flavors & Fragrances
14 de agosto de 2013
Hello Mr. Undersander, very interesting piece of paper and even though I consider myself illiterate in this matter, I wish to ask you the following: I would assume that phytate, as another nutrient in raw materials can eventually be NIR-analyzed; following this assumption, how reliable NIR can be when determining the content of phytate in an ingredient and how different could the different equations be when comparing ingredient to ingredient?. Many thanks in advance and congratulations on your work. Rafa from Madrid, Spain....in a very hot day.
7 de octubre de 2013
Good Day Prof Undersander. Congratulations for this emerging technology. Am interested in fish nutrition and I will like to find out if this technology can be used in fish nutrition research. how can those of us in Nigeria get to acquire the skills on how to use the NIR. what is the cost?
Dinamica Generale
27 de agosto de 2013
Good Day Mr Undersander, a quick question for you. As you write in your article "The big advantages of NIR are that it is much less expensive and more repeatable. Therefore a farmer would get better results by running multiple samples by NIR than a single sample by wet chemistry when the cost would be the same or less.", why in your opinion are farmers still so reluctant in buying Nir devices for real time analysis, when it is so evident the benefit at different level for their operation? Thanks in advance a greetings from Manuela - Italy










.jpg&w=3840&q=75)