Digestion and Absorption in Ruminants
Symposium: lonophores and Nutrient Digestion and Absorption in Ruminants
Published: September 20, 2010
By: Jerry W. Spears (Department of Animal Science, North Carolina State University)
This paper reviews the effects of feeding ionophores on nutrient digestion and absorption. In cattle, monensin and lasalocid increase apparent digestible energy by an average of 2.0 percentage units. In sheep, responses in digestible energy to ionophore feeding have been more variable, and neither monensin nor lasalocid have, on average, affected digestible energy. The effect of ionophores on fiber digestibility appears to depend on diet composition and source of fiber because both increases and decreases in fiber digestibility have been associated with ionophore feeding. Lasalocid and monensin reduce the percentage of starch digested in the rumen and increase the quantity of starch digested in the intestine. However, total gastrointestinal tract digestion of starch has generally not been affected by ionophores. Apparent nitrogen digestibility has been increased by ionophore feeding in a number of animal species. Apparent absorption of magnesium, phosphorus, zinc and selenium has been increased by ionophore supplementation. Absorption of calcium, potassium and sodium has been inconsistently affected by ionophores. Possible mechanisms whereby ionophores may affect nutrient digestion and absorption are discussed. J. Nutr. 120:632-638, 1990
Monensin and lasalocid are ionophores extensively used in ruminant diets to improve efficiency and/or rate of gain. In poultry, ionophores are widely used as anticoccidial agents. lonophores are compounds that form lipid-soluble complexes with certain cations and facilitate their transport across biological membranes (1). The beneficial effects of feeding ionophores to animals can be attributed to their ability to manipulate ion transport.
lonophores differ in their affinity and binding selectivity for cations. The ionophores that will be discussed in this paper include monensin, lasalocid, salinomycin and lysocellin. These compounds are approved for use in ruminants or have been investigated and shown to produce performance responses in ruminants. Monensin has a strong preference for sodium over potassium and does not bind divalent ions to any extent (2).Salino mycin has a slightly greater affinity for potassium than sodium, but it has little affinity for divalent ions (3). Lasalocid and lysocellin have affinities for divalent cat ions in addition to the monovalent cations, sodium and potassium (4).
Improvements in animal performance associated with ionophore feeding in ruminants have been largely attributed to alterations in ruminai microbial metabo lism (5, 6). When ionophores are fed, the digestive tract of the animal is exposed to the ionophore until it is either absorbed, excreted or modified so that it is no longer biologically active. Therefore, ionophores may affect digestion and/or absorption of nutrients in the rumen or small and large intestine. In isolated cells, ionophores increase cellular sodium levels, and this increases activity of the sodium-potassium pump (7, 8). Increases in sodium flux and the sodium-potassium pump in the gastrointestinal tract could affect the rate of absorption because active transport of a number of nutrients is coupled to sodium transport and the energy derived from the Na+/k+-ATPase (9). This paper will review the effects of ionophores on nutrient digestion and absorption.
TABLE 1
Click here to enlarge the image
lonophores differ in their affinity and binding selectivity for cations. The ionophores that will be discussed in this paper include monensin, lasalocid, salinomycin and lysocellin. These compounds are approved for use in ruminants or have been investigated and shown to produce performance responses in ruminants. Monensin has a strong preference for sodium over potassium and does not bind divalent ions to any extent (2).Salino mycin has a slightly greater affinity for potassium than sodium, but it has little affinity for divalent ions (3). Lasalocid and lysocellin have affinities for divalent cat ions in addition to the monovalent cations, sodium and potassium (4).
Improvements in animal performance associated with ionophore feeding in ruminants have been largely attributed to alterations in ruminai microbial metabo lism (5, 6). When ionophores are fed, the digestive tract of the animal is exposed to the ionophore until it is either absorbed, excreted or modified so that it is no longer biologically active. Therefore, ionophores may affect digestion and/or absorption of nutrients in the rumen or small and large intestine. In isolated cells, ionophores increase cellular sodium levels, and this increases activity of the sodium-potassium pump (7, 8). Increases in sodium flux and the sodium-potassium pump in the gastrointestinal tract could affect the rate of absorption because active transport of a number of nutrients is coupled to sodium transport and the energy derived from the Na+/k+-ATPase (9). This paper will review the effects of ionophores on nutrient digestion and absorption.
TABLE 1

Click here to enlarge the image
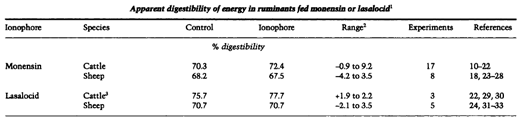
1 Organic matter or dry matter digestibility values were used when energy digestibility values were not available.
2 Range in percentage unit change in digestibility due to ionophore feeding.
3 Means for control and treated animals differ (p < 0.01} when analyzed by analysis of variance using experiment as replicate.
2 Range in percentage unit change in digestibility due to ionophore feeding.
3 Means for control and treated animals differ (p < 0.01} when analyzed by analysis of variance using experiment as replicate.
IONOPHORES AND ENERGY DIGESTION AND ABSORPTION
A major portion of the energy substrates in ruminant diets is fermented by ruminai microorganisms to vola tile fatty acids (VFA),methane and carbon dioxide. Volatile fatty acids produced by microbial fermentation are Absorbed and serve as a major energy source for the ruminant.
A summary of studies evaluating the influence of monensin and lasalocid on apparent digestible energy in cattle and sheep is presented in Table 1. In this summary, organic matter or dry matter digestibility values were used when digestible energy values were not available. This summary only includes studies that involved a total collection of feces and those in which feed intake did not differ statistically between control and ionophore-fed animals. In experiments with cattle, monensin and lasalocid increased apparent digestible energy by 2.0 percentage units on average. Responses to monensin feeding in various studies with cattle have ranged from a 0.9 percentage unit decrease (10) to a 9.2 percentage unit increase (11). A pooled statistical analysis using experiment as replicate and treatment means from each experiment for control and monensin-fed cattle indicated that the tendency for improved digestible energy was not significant (p < 0.16). Statistical analysis of cattle studies with lasalocid revealed an increase (p < 0.01) in apparent digestible energy of 2.0 percentage units in those fed lasalocid. This was due to the small variation in response between studies.
The response in digestible energy to ionophore feeding has been more variable in sheep, and neither monen sin nor lasalocid have, on average, affected digestible energy in sheep (Table 1). Differences between cattle and sheep in digestible energy responses to monensin and lasalocid may relate to species differences or differences in experimental diets. High concentrate diets were used in most cattle studies evaluating the effects of ionophores on digestion whereas high fiber diets were used in many of the sheep studies. In sheep and cattle fed high concentrate diets, ionophore feeding has either increased (11, 13, 14, 18, 20, 22, 31) or had no effect (10,15, 17, 19, 27, 29, 30) on energy, organic matter or drymatter digestibility. With high fiber diets, monensin feeding has depressed digestible organic matter in some studies (23, 25, 28).
Increases in digestible energy associated with ionophore feeding are not restricted to ruminants. Lasalocid at a level of 110 mg/kg diet increased digestible energy by 2.4 percentage units in growing pigs fed a corn-soybean-meal-based diet with 25% alfalfa (34). Salinomycin increased digestible energy by 1.5 percentage units in pigs fed a corn-soybean-meal diet that contained 20% wheat bran (35).
Of the energy-supplying nutrients, fiber can explain much of the variation in the response of digestible energy to ionophore feeding. The effect of ionophores on fiber digestibility appears to depend on diet composition and source of fiber. In ruminants fed high concentrate diets, fiber digestibility has frequently been increased by monensin (11, 13,14,18, 26) and lasalocid (31). Increases in fiber digestibility due to ionophore feeding also have been noted in cattle fed high fiber diets (18, 21, 37).
Monensin increased fiber digestibility in steers fed cornstalk-based diets (21), and monensin (37) and salinomycin (38) increased fiber digestibility in steers grazing forage. Fiber digestibility was not affected by monensin in steers fed orchardgrass hay (16) or by monensin or lysocellin in steers fed tall fescue greenchop (12).
A major portion of the energy substrates in ruminant diets is fermented by ruminai microorganisms to vola tile fatty acids (VFA),methane and carbon dioxide. Volatile fatty acids produced by microbial fermentation are Absorbed and serve as a major energy source for the ruminant.
A summary of studies evaluating the influence of monensin and lasalocid on apparent digestible energy in cattle and sheep is presented in Table 1. In this summary, organic matter or dry matter digestibility values were used when digestible energy values were not available. This summary only includes studies that involved a total collection of feces and those in which feed intake did not differ statistically between control and ionophore-fed animals. In experiments with cattle, monensin and lasalocid increased apparent digestible energy by 2.0 percentage units on average. Responses to monensin feeding in various studies with cattle have ranged from a 0.9 percentage unit decrease (10) to a 9.2 percentage unit increase (11). A pooled statistical analysis using experiment as replicate and treatment means from each experiment for control and monensin-fed cattle indicated that the tendency for improved digestible energy was not significant (p < 0.16). Statistical analysis of cattle studies with lasalocid revealed an increase (p < 0.01) in apparent digestible energy of 2.0 percentage units in those fed lasalocid. This was due to the small variation in response between studies.
The response in digestible energy to ionophore feeding has been more variable in sheep, and neither monen sin nor lasalocid have, on average, affected digestible energy in sheep (Table 1). Differences between cattle and sheep in digestible energy responses to monensin and lasalocid may relate to species differences or differences in experimental diets. High concentrate diets were used in most cattle studies evaluating the effects of ionophores on digestion whereas high fiber diets were used in many of the sheep studies. In sheep and cattle fed high concentrate diets, ionophore feeding has either increased (11, 13, 14, 18, 20, 22, 31) or had no effect (10,15, 17, 19, 27, 29, 30) on energy, organic matter or drymatter digestibility. With high fiber diets, monensin feeding has depressed digestible organic matter in some studies (23, 25, 28).
Increases in digestible energy associated with ionophore feeding are not restricted to ruminants. Lasalocid at a level of 110 mg/kg diet increased digestible energy by 2.4 percentage units in growing pigs fed a corn-soybean-meal-based diet with 25% alfalfa (34). Salinomycin increased digestible energy by 1.5 percentage units in pigs fed a corn-soybean-meal diet that contained 20% wheat bran (35).
Of the energy-supplying nutrients, fiber can explain much of the variation in the response of digestible energy to ionophore feeding. The effect of ionophores on fiber digestibility appears to depend on diet composition and source of fiber. In ruminants fed high concentrate diets, fiber digestibility has frequently been increased by monensin (11, 13,14,18, 26) and lasalocid (31). Increases in fiber digestibility due to ionophore feeding also have been noted in cattle fed high fiber diets (18, 21, 37).
Monensin increased fiber digestibility in steers fed cornstalk-based diets (21), and monensin (37) and salinomycin (38) increased fiber digestibility in steers grazing forage. Fiber digestibility was not affected by monensin in steers fed orchardgrass hay (16) or by monensin or lysocellin in steers fed tall fescue greenchop (12).
The higher fiber digestibilities observed in ruminants fed ionophores may result from a longer solids retention time in the rumen, thus allowing greater time for microbial digestion of fiber. Monensin reduced solids turnover in the rumen by 44% in steers fed low quality range grass (39) and reduced total tract turnover by -10% in steers grazing bermudagrass pasture (37). Solids turnover in the rumen of lambs fed an alfalfa-corn-based diet tended to be decreased by both monensin and lasalocid (24). Kobayashi et al. (40) reported that salinomycin reduced solids turnover by 24% in the rumen and by 25% in the large intestine. A slower rate of fiber digestion in the rumen may explain the slower rumen turnover. However, in situ studies indicated that the rate of ruminai cellulose disappearance is not affected by monensin or lasalocid (21, 24, 39).
lonophores have decreased or at least tended to reduce fiber digestibility in some studies (23, 28, 41). Monensin reduced fiber digestibility in lambs fed corn cobs as a source of fiber (28). Also, monensin decreased dry matter digestibility in lambs fed a high fiber diet containing 50% cottonseed hulls (25). In lambs fed a corn silage-based diet, monensin also depressed fiber digestibility (23). Monensin (40 mg/kg) reduced neutral detergent fiber digestibility from 79 to 8% in hamsters fed a semipurified diet containing cellulose as the fiber source (42). In the same experiment, adding monensin to a conventional diet containing alfalfa as the fiber source did not affect neutral detergent fiber digestibility. Adding monensin at a level of 1 ug/mL to ruminai microorganisms not adapted to monensin almost totally inhibited in vitro cellulose digestion when barley straw and cotton fibers were used as the substrate (43). However, in vitro cellulose digestion was not greatly inhibited by the same concentration of monensin when ryegrass or powdered filter paper was used as a substrate(43). These results suggest that chemical and/or physical properties associated with different fiber sources influence fiber digestibility response to ionophores.
Total gastrointestinal tract digestion of starch generally has not been affected by ionophores (10, 14, 15, 22,31, 44). However, lasalocid (31) and monensin (10) reduce the percentage of starch digested in the rumen and increase the quantity of starch digested in the intestine.
lonophores have decreased or at least tended to reduce fiber digestibility in some studies (23, 28, 41). Monensin reduced fiber digestibility in lambs fed corn cobs as a source of fiber (28). Also, monensin decreased dry matter digestibility in lambs fed a high fiber diet containing 50% cottonseed hulls (25). In lambs fed a corn silage-based diet, monensin also depressed fiber digestibility (23). Monensin (40 mg/kg) reduced neutral detergent fiber digestibility from 79 to 8% in hamsters fed a semipurified diet containing cellulose as the fiber source (42). In the same experiment, adding monensin to a conventional diet containing alfalfa as the fiber source did not affect neutral detergent fiber digestibility. Adding monensin at a level of 1 ug/mL to ruminai microorganisms not adapted to monensin almost totally inhibited in vitro cellulose digestion when barley straw and cotton fibers were used as the substrate (43). However, in vitro cellulose digestion was not greatly inhibited by the same concentration of monensin when ryegrass or powdered filter paper was used as a substrate(43). These results suggest that chemical and/or physical properties associated with different fiber sources influence fiber digestibility response to ionophores.
Total gastrointestinal tract digestion of starch generally has not been affected by ionophores (10, 14, 15, 22,31, 44). However, lasalocid (31) and monensin (10) reduce the percentage of starch digested in the rumen and increase the quantity of starch digested in the intestine.
This shift in site of digestion should result in more carbon from starch being absorbed as glucose rather than as VFA and, thus, should enable more efficient use of energy. Monensin also may increase the enzymatic capability for starch digestion in the small intestine. Higher amylase activities were found in the pancreas (45) and feces (20) of cattle receiving monensin.
Changes in digestible energy induced by feeding ionophores should alter the quantities of VFA, and perhaps glucose, available for absorption. Monensin increases ruminal propionate production (46-48), allowing for greater propionate absorption. In addition, Rogers and Davis (48) reported that monensin increased the amount of ruminal acetate produced per kilogram dry matter intake by steers. Salinomycin and monensin increased net portal flux of propionate in cattle fed a high concentrate diet (49). In a second study (50), net portal flux of butyrate was decreased, but propionate was not affected by monensin in steers fed a similar high concentrate diet. Net portal flux of propionate and acetate decreased following monensin removal in steers fed alfalfa hay (49). Increased portal blood concentrations of propionate were observed in pigs fed salinomycin (51).T he effect of ionophores on the rate of absorption of the major end products of carbohydrate digestion, VFA and glucose, has not been examined.
TABLE 2
Changes in digestible energy induced by feeding ionophores should alter the quantities of VFA, and perhaps glucose, available for absorption. Monensin increases ruminal propionate production (46-48), allowing for greater propionate absorption. In addition, Rogers and Davis (48) reported that monensin increased the amount of ruminal acetate produced per kilogram dry matter intake by steers. Salinomycin and monensin increased net portal flux of propionate in cattle fed a high concentrate diet (49). In a second study (50), net portal flux of butyrate was decreased, but propionate was not affected by monensin in steers fed a similar high concentrate diet. Net portal flux of propionate and acetate decreased following monensin removal in steers fed alfalfa hay (49). Increased portal blood concentrations of propionate were observed in pigs fed salinomycin (51).T he effect of ionophores on the rate of absorption of the major end products of carbohydrate digestion, VFA and glucose, has not been examined.
TABLE 2
Click here to enlarge the image
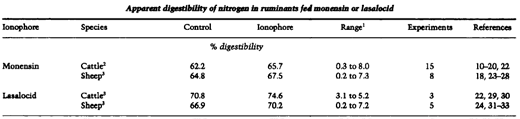
1 Range in percentage unit change in digestibility due to ionophore feeding.
2 Means for control and treated animals differ (p< 0.01) when analyzed by analysis of variance using experiment as replicate.
3 Means for control and treated animals differ (p< 0.05) when analyzed by analysis of variance using experiment as replicate.
IONOPHORES AMD NITROGEN DIGESTION AND ABSORPTION
Studies in cattle (10-14, 22, 29, 41,44), sheep (18, 24, 26, 32, 45), goats (13), hamsters (42) and pigs (34, 35) indicate higher apparent nitrogen digestibilities in animals fed ionophores. Table 2 shows a summary of studies in which apparent nitrogen digestibility was measured in cattle and sheep fed monensin or lasalocid. A pooled statistical analysis using experiment as replicate and treatment means from each experiment was conducted. Significant increased in apparent nitrogen digestibility were noted with both ionophores in cattle and sheep. The increase in apparent nitrogen digestibility averaged between 2.6 and 3.8 percentage units, with cattle showing a slightly greater response than sheep.
Lysocellin increased apparent nitrogen digestibility in steers fed greenchop, with the increase in digestibility being similar to that noted in steers fed monensin (12). Increases in apparent digestibility of nitrogen also were observed in sheep (44)and pigs (35) fed salinomycin.
A higher ratio of dietary to microbial protein entering the small intestine may explain the higher apparent nitrogen digestibility in ruminants fed ionophores (44). Monensin reduces ruminal degradation of preformed dietary protein and decreases microbial protein flow to the small intestine (5, 28). Feed protein is usually more digestible than microbial protein (52). This phenomenon cannot explain the increased nitrogen digestibility observed in pigs fed ionophores (34, 35).
Fecal endogenous nitrogen losses may be reduced by ionophore feeding, and this may at least partially explain the higher apparent nitrogen digestibilities. Reductions in fecal endogenous nitrogen could arise from decreased microbial protein synthesis in the large intestine and cecum or from decreased sloughing of intestinal cells. Salinomycin decreased fecal microbial nitrogen output in pigs, suggesting reduced microbial protein synthesis in the lower gastrointestinal tract (53).
Ionophores may affect the rate of amino acid absorption. Most amino acids are absorbed via a sodium co-transport mechanism, and the energy required for transport is provided by the Na+/K+-ATPase (9). Ionophores increase cellular sodium and increase activity of the sodium-potassium pump (7, 8). Esteve-Garcia et al. (54) examined absorption of [14C] amino acids over a 5-min period from ligated segments of small intestine from chicks fed a control or a monensin-supplemented (121 mg/kg) diet. Dietary monensin increased absorption of arginine and tryptophan by 69 and 34%, respectively, but decreased absorption of lysine by 13%. The addition of monensin to the 14C-labeled amino acid solution decreased absorption of lysine, threonine and isoleucine but increased absorption of arginine in chicks fed the control diet.
Studies in cattle (10-14, 22, 29, 41,44), sheep (18, 24, 26, 32, 45), goats (13), hamsters (42) and pigs (34, 35) indicate higher apparent nitrogen digestibilities in animals fed ionophores. Table 2 shows a summary of studies in which apparent nitrogen digestibility was measured in cattle and sheep fed monensin or lasalocid. A pooled statistical analysis using experiment as replicate and treatment means from each experiment was conducted. Significant increased in apparent nitrogen digestibility were noted with both ionophores in cattle and sheep. The increase in apparent nitrogen digestibility averaged between 2.6 and 3.8 percentage units, with cattle showing a slightly greater response than sheep.
Lysocellin increased apparent nitrogen digestibility in steers fed greenchop, with the increase in digestibility being similar to that noted in steers fed monensin (12). Increases in apparent digestibility of nitrogen also were observed in sheep (44)and pigs (35) fed salinomycin.
A higher ratio of dietary to microbial protein entering the small intestine may explain the higher apparent nitrogen digestibility in ruminants fed ionophores (44). Monensin reduces ruminal degradation of preformed dietary protein and decreases microbial protein flow to the small intestine (5, 28). Feed protein is usually more digestible than microbial protein (52). This phenomenon cannot explain the increased nitrogen digestibility observed in pigs fed ionophores (34, 35).
Fecal endogenous nitrogen losses may be reduced by ionophore feeding, and this may at least partially explain the higher apparent nitrogen digestibilities. Reductions in fecal endogenous nitrogen could arise from decreased microbial protein synthesis in the large intestine and cecum or from decreased sloughing of intestinal cells. Salinomycin decreased fecal microbial nitrogen output in pigs, suggesting reduced microbial protein synthesis in the lower gastrointestinal tract (53).
Ionophores may affect the rate of amino acid absorption. Most amino acids are absorbed via a sodium co-transport mechanism, and the energy required for transport is provided by the Na+/K+-ATPase (9). Ionophores increase cellular sodium and increase activity of the sodium-potassium pump (7, 8). Esteve-Garcia et al. (54) examined absorption of [14C] amino acids over a 5-min period from ligated segments of small intestine from chicks fed a control or a monensin-supplemented (121 mg/kg) diet. Dietary monensin increased absorption of arginine and tryptophan by 69 and 34%, respectively, but decreased absorption of lysine by 13%. The addition of monensin to the 14C-labeled amino acid solution decreased absorption of lysine, threonine and isoleucine but increased absorption of arginine in chicks fed the control diet.
TABLE 3
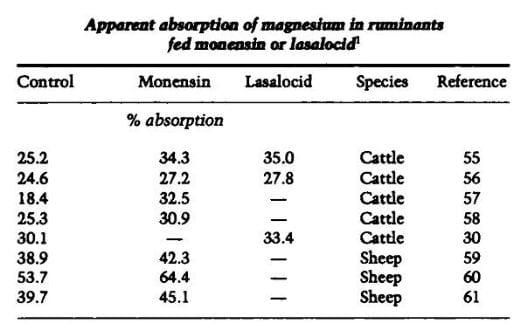
'Summary of studies in which magnesium apparent absorption has been measured in ruminants receiving monensin or lasalocid.

'Summary of studies in which magnesium apparent absorption has been measured in ruminants receiving monensin or lasalocid.
TABLE 4
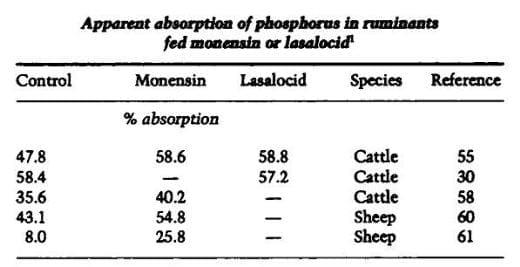
'Summary of studies in which phosphorus absorption has been measured in ruminants receiving monensin or lasalocid.
IONOPHORES AND MINERAL ABSORPTION
Numerous studies indicate that ionophores affect apparent absorption of certain minerals. Results have been inconsistent, suggesting that the effect of ionophores on mineral absorption is affected by dietary and/or physiological factors.
Apparent absorption of magnesium has been consistently increased in ruminants by monensin and lasalocid (Table 3). Significant increases in magnesium absorption were observed in steers and lambs fed high concentrate diets (55-57, 60, 61) and in steers fed tall fescue greenchop (58). Lysocellin also increased magnesium absorption in steers fed greenchop (58) and corn silage-based diets (62). Using steers with proximal duodenal and terminal ileal cannulas, Greene et al. (57) found that the increase in magnesium absorption with monensin feeding resulted from increased preintestinal absorption. The rumen is the major site of magnesium absorption in ruminants (63). The effects of monensin on magnesium absorption may not be confined to the rumen because O'Conner and Beede (64) found that monensin increased in vitro magnesium absorption by bovine duodenal tissue. Using steers fitted with duodenal and ileal cannulas, Gado et al. (56) found that monensin and lasalocid increased magnesium absorption from the small intestine. Salinomycin has not affected magnesium absorption in steers (30) or in pigs (65).
Apparent absorption of phosphorus also was increased by monensin and lasalocid in most experiments (Table4). Lysocellin also increased phosphorus absorption in steers (58). Salinomycin increased apparent absorption of phosphorus in pigs (65) but not in steers (30). Apparent absorption of calcium has been increased by monensin (56,58,60,61), lasalocid (56) and lysocellin (58) in ruminants. In other studies, apparent absorption of calcium was not affected by ionophore feeding (57, 59). Apparent absorption of calcium was much lower in steers fed salinomycin compared to steers fed lasalocid or no ionophore (30). A tendency for lower apparent absorption of calcium in steers fed salinomycin also was noted by Zinn (66).
Apparent absorptions of potassium and sodium have not been consistently affected by ionophore feeding, lonophores increased potassium absorption in some studies (30, 57, 58, 67) but not in others (30). In most studies (60-62,67), sodium apparent absorption was not affected by ionophore feeding. However, Starnes et al. (55) reported that monensin and lasalocid increased sodium apparent absorption in steers fed a high concentrate diet. In contrast, both monensin and lysocellin reduced apparent absorption of sodium in steers fed greenchop (58).
lonophores also affect the metabolism of certain trace elements. In steers fed a hay-straw diet adequate in zinc (26.4 mg/kg) but deficient in selenium (0.026 mg/kg), monensin increased whole-body retention of a single oral dose of 65 Zn and 75 Se by 43 and 75%, respectively (68). Retention of zinc and selenium was measured over a 14-d period after steers had received monensin for 21 to 28 d. The increased selenium retention by steers fed monensin supports research in which monensin supple mentation increased whole blood glutathione peroxidase, a selenium metalloenzyme, in ewes fed a selenium-deficient diet (69). Apparent absorption of zinc also was increased by monensin in lambs (59) and by salinomycin in steers (66).
Copper absorption has not been measured in ruminants fed ionophores; however, based on plasma copper concentrations, ionophores appear to increase copper absorption under certain conditions. Plasma copper concentrations were 33% higher at 28 d and 62% higher at 90 d in steers fed supplemental salinomycin or lasalocid compared to controls fed a corn silage-based diet marginal in copper (30). Plasma copper decreased between 28 and 90 d in control steers but not in ionophore-supplemented steers. lonophores may affect copper absorption by reducing ruminal protozoa numbers or by decreasing ruminal production of sulfide from sulfur amino acids. Some studies (28, 70) have indicated lower protozoa numbers in ruminants fed ionophores. Ivan etal. (71) observed much higher liver and plasma copper concentrations in fauna-free compared to faunated lambs. Reductions in ruminal degradation of dietary protein caused by ionophores (5) could result in lower ruminal sulfide concentrations. Evidence suggests that sulfide is the key form of sulfur for reducing copper absorption in ruminants (72). Changes in apparent mineral absorption associated with monensin feeding appear to occur rapidly. In a recent study (61), mineral absorption was measured for the first 7 d (unadapted) following initiation of monensin feeding, and a second 7-d collection was conducted beginning on d 29 (adapted). Compared to controls, unadapted lambs fed monensin had higher apparent absorption of phosphorus, magnesium and potassium. Calcium was the only mineral for which apparent absorption was increased by monensin in adapted but not in unadapted lambs.
Despite differences in their affinity and binding selectivity for cations, monensin, lasalocid and lysocellin have generally affected mineral absorption similarly. Changes in apparent absorption of minerals with lysocellin were similar to those observed in steers fed monensin (58). Lasalocid and monensin also have produced similar alterations in mineral absorption (55, 56). Salinomycin, however, appears to influence mineral absorption differently from lasalocid, especially in regard to calcium.
Mechanisms by which ionophores affect mineral absorption have not been elucidated. In addition to actually forming a complex with and altering transport of a given cation, ionophores may indirectly affect absorption of minerals through a coupled transport mechanism involving sodium and potassium or through changes in energy available for ion transport via altered monovalent ion gradients (73). Increased magnesium absorption associated with ionophore feeding may result from increased Na+/K+-ATPase activity (55, 59). Alterations in sodium transport and Na+/K+-ATPase activity also may explain the higher apparent absorption of phosphorus in ruminants fed ionophores, because intestinal phosphate absorption is linked to sodium and the Na+/K+-ATPase system (74). Finally, ionophores could affect mineral absorption by reducing coccidial numbers in the intestine. Inoculation of chicks with the coccidium Eimeria acervulina resulted in major changes in trace element metabolism (75).
In summary, ionophore supplementation consistently increased apparent digestibility of nitrogen in a number of animal species. Monensin and lasalocid in creased digestible energy by 2.0 percentage units when averaged across all studies with cattle. Responses in digestible energy to ionophore feeding in sheep has been more variable. Increases in energy and/or nitrogen digestibility can partially explain the improvements in efficiency observed in ruminants fed ionophores. Ionophore supplementation increased apparent absorption of magnesium, phosphorus, zinc and selenium whereas absorption of calcium, potassium and sodium has been affected inconsistently by ionophore feeding. Changes in mineral absorption associated with ionophore feeding may affect requirements for certain minerals.
NOTES

'Summary of studies in which phosphorus absorption has been measured in ruminants receiving monensin or lasalocid.
IONOPHORES AND MINERAL ABSORPTION
Numerous studies indicate that ionophores affect apparent absorption of certain minerals. Results have been inconsistent, suggesting that the effect of ionophores on mineral absorption is affected by dietary and/or physiological factors.
Apparent absorption of magnesium has been consistently increased in ruminants by monensin and lasalocid (Table 3). Significant increases in magnesium absorption were observed in steers and lambs fed high concentrate diets (55-57, 60, 61) and in steers fed tall fescue greenchop (58). Lysocellin also increased magnesium absorption in steers fed greenchop (58) and corn silage-based diets (62). Using steers with proximal duodenal and terminal ileal cannulas, Greene et al. (57) found that the increase in magnesium absorption with monensin feeding resulted from increased preintestinal absorption. The rumen is the major site of magnesium absorption in ruminants (63). The effects of monensin on magnesium absorption may not be confined to the rumen because O'Conner and Beede (64) found that monensin increased in vitro magnesium absorption by bovine duodenal tissue. Using steers fitted with duodenal and ileal cannulas, Gado et al. (56) found that monensin and lasalocid increased magnesium absorption from the small intestine. Salinomycin has not affected magnesium absorption in steers (30) or in pigs (65).
Apparent absorption of phosphorus also was increased by monensin and lasalocid in most experiments (Table4). Lysocellin also increased phosphorus absorption in steers (58). Salinomycin increased apparent absorption of phosphorus in pigs (65) but not in steers (30). Apparent absorption of calcium has been increased by monensin (56,58,60,61), lasalocid (56) and lysocellin (58) in ruminants. In other studies, apparent absorption of calcium was not affected by ionophore feeding (57, 59). Apparent absorption of calcium was much lower in steers fed salinomycin compared to steers fed lasalocid or no ionophore (30). A tendency for lower apparent absorption of calcium in steers fed salinomycin also was noted by Zinn (66).
Apparent absorptions of potassium and sodium have not been consistently affected by ionophore feeding, lonophores increased potassium absorption in some studies (30, 57, 58, 67) but not in others (30). In most studies (60-62,67), sodium apparent absorption was not affected by ionophore feeding. However, Starnes et al. (55) reported that monensin and lasalocid increased sodium apparent absorption in steers fed a high concentrate diet. In contrast, both monensin and lysocellin reduced apparent absorption of sodium in steers fed greenchop (58).
lonophores also affect the metabolism of certain trace elements. In steers fed a hay-straw diet adequate in zinc (26.4 mg/kg) but deficient in selenium (0.026 mg/kg), monensin increased whole-body retention of a single oral dose of 65 Zn and 75 Se by 43 and 75%, respectively (68). Retention of zinc and selenium was measured over a 14-d period after steers had received monensin for 21 to 28 d. The increased selenium retention by steers fed monensin supports research in which monensin supple mentation increased whole blood glutathione peroxidase, a selenium metalloenzyme, in ewes fed a selenium-deficient diet (69). Apparent absorption of zinc also was increased by monensin in lambs (59) and by salinomycin in steers (66).
Copper absorption has not been measured in ruminants fed ionophores; however, based on plasma copper concentrations, ionophores appear to increase copper absorption under certain conditions. Plasma copper concentrations were 33% higher at 28 d and 62% higher at 90 d in steers fed supplemental salinomycin or lasalocid compared to controls fed a corn silage-based diet marginal in copper (30). Plasma copper decreased between 28 and 90 d in control steers but not in ionophore-supplemented steers. lonophores may affect copper absorption by reducing ruminal protozoa numbers or by decreasing ruminal production of sulfide from sulfur amino acids. Some studies (28, 70) have indicated lower protozoa numbers in ruminants fed ionophores. Ivan etal. (71) observed much higher liver and plasma copper concentrations in fauna-free compared to faunated lambs. Reductions in ruminal degradation of dietary protein caused by ionophores (5) could result in lower ruminal sulfide concentrations. Evidence suggests that sulfide is the key form of sulfur for reducing copper absorption in ruminants (72). Changes in apparent mineral absorption associated with monensin feeding appear to occur rapidly. In a recent study (61), mineral absorption was measured for the first 7 d (unadapted) following initiation of monensin feeding, and a second 7-d collection was conducted beginning on d 29 (adapted). Compared to controls, unadapted lambs fed monensin had higher apparent absorption of phosphorus, magnesium and potassium. Calcium was the only mineral for which apparent absorption was increased by monensin in adapted but not in unadapted lambs.
Despite differences in their affinity and binding selectivity for cations, monensin, lasalocid and lysocellin have generally affected mineral absorption similarly. Changes in apparent absorption of minerals with lysocellin were similar to those observed in steers fed monensin (58). Lasalocid and monensin also have produced similar alterations in mineral absorption (55, 56). Salinomycin, however, appears to influence mineral absorption differently from lasalocid, especially in regard to calcium.
Mechanisms by which ionophores affect mineral absorption have not been elucidated. In addition to actually forming a complex with and altering transport of a given cation, ionophores may indirectly affect absorption of minerals through a coupled transport mechanism involving sodium and potassium or through changes in energy available for ion transport via altered monovalent ion gradients (73). Increased magnesium absorption associated with ionophore feeding may result from increased Na+/K+-ATPase activity (55, 59). Alterations in sodium transport and Na+/K+-ATPase activity also may explain the higher apparent absorption of phosphorus in ruminants fed ionophores, because intestinal phosphate absorption is linked to sodium and the Na+/K+-ATPase system (74). Finally, ionophores could affect mineral absorption by reducing coccidial numbers in the intestine. Inoculation of chicks with the coccidium Eimeria acervulina resulted in major changes in trace element metabolism (75).
In summary, ionophore supplementation consistently increased apparent digestibility of nitrogen in a number of animal species. Monensin and lasalocid in creased digestible energy by 2.0 percentage units when averaged across all studies with cattle. Responses in digestible energy to ionophore feeding in sheep has been more variable. Increases in energy and/or nitrogen digestibility can partially explain the improvements in efficiency observed in ruminants fed ionophores. Ionophore supplementation increased apparent absorption of magnesium, phosphorus, zinc and selenium whereas absorption of calcium, potassium and sodium has been affected inconsistently by ionophore feeding. Changes in mineral absorption associated with ionophore feeding may affect requirements for certain minerals.
NOTES
1- Presented as part of the 30th Annual Ruminant Nutrition Conference: Influence of Gut Metabolism on Nutrient Supply, given at the 73rd Annual Meeting of the Federation of American Societies for Experimental Biology, New Orleans, LA, March 19, 1989, and supported by grants from American Cyanamid Company; Cargill,Nutrena Feed Division; Carol S. Akey, Inc.; Monsanto; Pioneer HiBred International, Inc.; Pitman-Moore, Inc.; and SmithKline Beckman Animal Health Products.
2- Paper no. 12,437 of the Journal Series of the North Carolina Agricultural Research Services, Raleigh, NC 27695-7643. Use of trade names in this publication does not imply endorsement by the North Carolina Agricultural Research Service or criticism of similar products not mentioned.
3- Guest editor for this symposium was K. E. Webb, Jr., Department of Animal Science, Virginia Polytechnic Institute and State University, Blacksburg, VA.
LITERATURE CITED
1. PRESSMAN, B. C. (1976) Biological applications of ionophores.Ann. Rev. Biochem. 45: 501-530.
2. PRESSMANB,. C. & FAHIM,M. (1982) Pharmacology and toxicology of the monovalent carboxylic ionophores. Ann. Rev. Pharmacol.Toxicol. 22: 465-490.
3. MTTANIM, ., YAMANISHTI,. & MIYAZAKIY, . (1975) Salinomycin: a new monovalent cation ionophore. Biochem. Biophys. Res.Commun. 66: 1231-1236.
4. PAINTER,G. R. & PRESSMAN, B. C. (1985) Cation complexes of the monovalent and polyvalent carboxylic ionophores: lasalocid(X-537A), monensin, A23187 (calcimycin), and related antibiotics. In: Metal Ions in Biological Systems (Sigel, H., ed.), vol. 19,pp. 229-294, Marcel Dekker, New York, NY.
5. BERGEN,W. G. & BATES, D. B. (1984) Ionophores: their effect on production efficiency and mode of action. /. Anim. Sci. 58: 1465-1483.6. SCHELLINGG,. T. (1984) Monensin mode of action in the rumen. /. Anim. Sci. 58: 1518-1527.
7. AUSTIC,R. E. & SMTTH,J. B. (1980) Interaction of ionophores with nutrients. Proc. Georgia Nutr. Con/., pp. 2-10, University of Georgia, Athens, GA.
8. SMITH,J. B. & AUSTIC,R. E. (1980) Activating the Na-K pump with monensin increases aminoisobutyric acid uptake by mouse fibroblasts. Biochem. Biophys. Res. Commun. 93: 392-398.
9. GUYTON,A. C. (1986) Textbook of Medical Physiology, W. B.Saunders, Philadelphia, PA.
10. MuNTffERiNG,R. B., THEURER,B. & NOON, T. H. (1981) Effects of monensin on site and extent of whole corn digestion and bacterial protein synthesis in beef steers. /. Anim. Sci. 53: 1565-573.
11. HORTON,G. M. J. (1980) A note on the effect of monensin and amicloral in steer diets. Anim. Prod. 30: 441-444.
12. SPEARS,I. W., BURNS,J. C. & WOLFROM, G. W. (1989) Lysocellin effects on growth performance, ruminai fermentation, nutrient digestibility and nitrogen metabolism in steers fed forage diets. J. Anim. Sci. 67: 547-556.
13. BEEDE,D. K., SCHELLINGG, . T., MITCHELLG, . E., TUCKER,R. E., GILL, W. W., KOENIG,S. E. & LINDSEY,T. O. (1986) Nitrogen utilization and digestibility by growing steers and goats of diets that contain monensin and low crude protein. /. Anim. Sci. 62: 857-863.
14. WEDEGAERTNETR.,C. & JOHNSOND, . E. (1983) Monensin effects on digestibility, methanogenesis and heat increment of a cracked corn silage diet fed to steers. /. Anim. Sci. 57: 168-177.
15. MuNTffERiNG,R. B., THEURER,B., SWINGLE, R. S. & HALE,W. H. (1980) Effect of monensin on nitrogen utilization and digestibility of concentrate diets by steers. /. Anim. Sci. 50: 930-936.
16. DINIUS,D. A., SIMPSON,M. E. & MARSH,P. B. (1976) Effect of monensin fed with forage on digestion and the ruminai ecosystem of steers. /. Anim. Sci. 42: 229-234.
17. THORNTON,J. H. & OWENS,F. M. (1981) Monensin supplementation and in vivo methane production by steers. /. Anim. Sci. 52:628-634.
18. HORTON,G. M. J., BASSENDOWS&KIKELLERE, . H. (1980| Digestion and metabolism in lambs and steers fed monensin with different levels of barley. /. Anim. Sci. 50: 997-1008.
19. TOLBERTR, . E. & LICHTENWALNERR.,E. (1978) Effect of monen sin on apparent digestibility and nitrogen utilization of sorghum based rations. /. Anim. Sci. 47 (Suppl. 1): 276 (abs.).
20. ILAN,D., BEN-ASHER, A., HOLZER,Z., NITSAN,Z., NIR, I. & LEVY,D. (1981) Effect of monensin supplementation on growth, feed digestibility and utilization in young calves. Anim. Prod. 32: 125-131.
21. FAULKNERD,. B., KLOPFENSTEITN., J., TROTTER,T. N. & BRITTON,R. A. (1985) Monensin effects on digestibility, ruminai protein escape and microbial protein synthesis on high fiber diets. /. Anim. Sci. 61: 654-660.
22. FERRELLM, . C, GILL,D. R. & OWENSF, .N. (1982) Ionophores for feedlot steers. /. Anim. Sci. 55 (Suppl. 1): 421 (abs.).
23. COTTYN,B. G., FIEMS,L. O., BOUCQUE, C. V.,AERTS,J. V.& BUYSSE, F.X. (1983) Effect of monensin-sodium and avoparcin on digestibility and rumen fermentation. Z. Tierphysiol. Tieremaehi. Futtermittelkd. 49: 277-286.
24. RICKE,S.C., BERGER, L.L., VANDERAAR,P.J.&FAHEY,G.G. (1984)Effects of lasalocid and monensin on nutrient digestion, metabolism and rumen characteristics in sheep. /. Anim. Sci. 58: 194- 202.
25. VIJCHULATAP.,, HENRY,P. R., AMMERMANC,. B., BECKERH, . N. &.PALMER, A. Z. (1980) Performance and tissue mineral composition of ruminants fed cage layer mature in combination with monensin. /. Anim. Sci. 50: 48-56.
26. JOYNERA, . E.,BROWNL, .J., FOGG,T.J.& Rossi, R.T. (1979) Effectof monensin on growth, feed efficiency and energy metabolism of lambs. /. Anim. Sci. 48: 1065-1069.
27. HORTON,G. M. J. (1980) Effect of monensin and a deaminase inhibitor on feed utilization by lambs. Can. J. Anim. Sci. 60: 169-172.
28. Poos, M. L, HANSON,T. L. & KLOPFENSTEITN.,I. (1979) Monensin effects on diet digestibility, ruminai protein bypass and microbial protein synthesis. /. Anim. Sci. 48: 1516-1524.
29. DELFINO,J., MATMSON,G. W. &. SMITH,M. W. (1988) Effect of lasalocid on feedlot performance and energy partitioning in cattle. /. Anim. Sci. 66: 136-150.
30. STABELJ,. R., SPEARSJ,.W., HARVEYR, . W. & LUCASD, . M. (1989) Salinomycin and lasalocid effects on growth performance, mineral metabolism and ruminai fermentation in steers. /. Anim. Sci. 67: 2735-2742.
31. FUNK,M. A., GALYEANM, . L. &Ross,T.T. (1986) Potassium and lasalocid effects on performance and digestion in lambs. /. Anim. Sci. 63:685-691.
32. PATERSONJ,. A., ANDERSONB, . M., BOWMAND, . K., MORRISONR, . L. & WILLIAMS]., E. (1983) Effect of protein source and lasalocid on nitrogen digestibility and growth by ruminants. /. Anim. Sci. 57: 1537-1544.
33. TmvEND, P. & JOUANY, J. P. (1983) Effect of lasalocid sodium on rumen fermentation and digestion in sheep. Reprod. Nutr. Dev.23:817-828.
34. COOK,D. A., FRALEYJ,. R., JENSENA, . H. & WEDEKINDK, . J. (1988) Effects of dietary lasalocid on diet utilization by growing-finish ing pigs. /. Anim. Sci. 66 (Suppl. 1): 132 (abs.).
35. MOORE,R. f., KORNEGAYE.,T. & LINDEMANNM, . D. (1986) Effect of Salinomycin on nutrient absorption and retention by growing pigs fed corn-soybean meal diets with or without oat hulls or wheat bran. Can. }. Anim. Sci. 66: 257-265.
36. HORTON,G. M. J. & NICHOLSON, H. H. (1980) Rumen metabo lism and feedlot responses by steers fed tylosin and monensin. Can. J. Anim. Sci. 60: 919-924.
37. ELLIS,W. C., MATIS,J. H., POND,K. R., LASCANO, C. E. & TELFORD, J. P. (1984) Dietary influences on flow rate and digestive capacity. In: Herbivore Nutrition in the Subtropics and Tropics (Gilchrist, F. M. C. & Mackie, R. I., eds.), pp. 269-293, The Science Press, Craighall, South Africa.
38. BARCLAYR,. A., FAULKNERD,. B., FAHEYG, . C. & CMARICKG, . F. (1986) Effects of Salinomycin on performance characteristics and apparent dry matter digestion by grazing beef steers. Nutr. Rep. Int. 33: 43-54.
39. LEMENAGERR,. P., OWENS,F. N., SHOCKEYB, . J., LUSBYK, . S. & TOTUSEK,R. (1978) Monensin effects on rumen turnover rate, twenty-four hour VFA pattern, nitrogen components and cellulose disappearance. /. Anim. Sci. 47: 255-261.
40. KOBAYASWY,, WAKTTAM, . & HOSHINO,S. (1986) Effects of salinomycin on digesta passage, digestibility, nitrogen balance and ruminai traits in wethers. /. Anim. Physiol. Anim. Nutr. 56:90-96.
41. WEBB,K. E., Jr., FONTENOT, J. P. &.LUCAS,D. M. (1980) Metabolism studies in steers fed different levels of salinomycin. /. Anim. Downloaded from jn.nutrition.org by on September 13, 2010 638 SPEARS Sci. 51 (Supplì):407 (abs.).
42. SAKAGUCM, E. & MATSUMOTO, T. |1985) Effect of monensin on feed utilization and gastrointestinal fermentation in the hamster. Bi. ]. ÑutÃ. 54: 147-155.
43. HENDERSONC,, STEWARTC,. S. & NEKREPF, .V. (1981) The effect of monensin on pure and mixed cultures of rumen bacteria. /. Appi. Bact. 51: 159-169.
44. MERCHEN,N. R. & BERCER,L. L. (1985) Effect of salinomycin level on nutrient digestibility and ruminai characteristics of sheep and feedlot performance of cattle. /. Anïm.Sci. 60: 1338-1346.
45. VANHELLEN,R. W., WILSON,T. A., BOLING,J. A, MITCHELL, G. E., TUCKER,R. E. & SCHELLING, G. T. (1977) Bovine amylase and protease response to monensin. /. Anim. Sci. 45 (Suppl. 1): 265 (abs.).
46. PRANCE,R. W., DAVIS,C. L. & CLARK,J. H. (1978) Propionate production in the rumen of Holstein steers fed either a control or monensin supplemented diet. /. Anim. Sci. 46: 1120-1124.
47. VANMAANEN,R. W., HERBEIN],. H., MCGILLIARDA, . D. & YOUNG, J. W. (1978) Effects of monensin on in vivo propionate production and blood glucose kinetics in cattle. /. Nutr. 108: 1002-1007.
48. ROGERS,J. A. & DAVIS,C. L. (1982) Rumen volatile fatty acid production and nutrient utilization in steers fed a diet supple mented with sodium bicarbonate and monensin. /. Dairy Sci. 65: 944-952.
49. HARMON,D. L., AVERYT, . B., HUNTINGTONG, . B. &.REYNOLDSP,. J. (1988) Influence of ionophore addition to roughage and high concentrate diets on portal blood flow and net nutrient flux in cattle. Can. J. Anim. Sci. 68: 419-429.
50. HARMON,D. L. & AVERY,T. B. (1987) Effects of dietary monensin and sodium propionate on net nutrient flux in steers fed a high concentrate diet. /. Anim. Sci. 65: 1610-1616.
51. MCMILLAN,E. &.MORAN,E. T. (1986) Salinomycin affects volatile fatty acid production and absorption from the large intestine of growing pigs. Fed. Proc. 45: 241 (abs.).
52. VANSOEST,P. J. (1982) Nutritional Ecology of the Ruminant. O&.B Books, Corvallis, OR.
53. DEWlLDE,R. O. (1984) Comparison of virginiamycin and salinomycin as growth promoters in growing-fattening pigs. Dtsch. Tieiaeiztl. Wochenschr. 91: 22-24.
54. ESTEVE-GARCIAE,., RILEY,W. W. & Ausnc, R. E. (1985) Effects of monensin on amino acid absorption in small intestine. Paul. Sci. 64 (Suppl. 1):97 (abs.).
55. STARNESS, . R., SPEARSJ,. W., FROETSCHELM,. A. & GROOM,W. J. (1984) Influence of monensin and lasalocid on mineral metabolism and ruminai urease activity in steers. /. Nutr. 114: 518-525.
56. GADO, H. M., GOODRICH,R. D., GARRETT,J. E. & MEISKE,J. C. (1986) Effects of concentrate level and ionophores on energy, protein and mineral digestion in steers. /. Anim. Sci. 63 (Suppl.1): 440 (abs.).
57. GREENEL, . W., MAY,B. J., SCHELLINGG,. T. &.BYERSF, .M. (1988) Site and extent of apparent magnesium and calcium absorption in steers fed monensin. /. Anim. Sci. 66: 2987-2991.
58. SPEARSJ,.W., SCHRICKERB,. R. & BURNSJ, . C. (1989) Influence of lysocellin and monensin on mineral metabolism of steers fed forage-based diets. /. Anim. Sci. 67: 2140-2149.
59. KIRK,D. J., GREENEL, .W., SCHELLINGG,. T. & BYERSF, .M. (1985) Effects of monensin on Mg, Ca, P and Zn metabolism and tissue concentrations in lambs. /. Anim. Sci. 60: 1485-1490.
60. GREENEL, .W., SCHELLINGG,. T. & BYERSF, .M. (1986) Effects of dietary monensin and potassium on apparent absorption of magnesium and other macroelements in sheep. /. Anim. Sci. 63:1960-1967.
61. DROKE,E. A., SPEARS,J. W., ARMSTRONG, J. D. & KEGLEY,E. B. (1989) Effect of feeding monensin on mineral metabolism in lambs unadapted and adapted to monensin. /. Anim. Sci. 67 (Suppl. 1): 567 (abs.).
62. KEGLEY,E. B., HARVEY,R. W. & SPEARS,I. W. (1989) Effect of lysocellin and calcium levels on mineral metabolism, performance and ruminai and plasma characteristics of beef steers. /Anim. Sci. 67 (Suppl. 2): 30 (abs.).
63. MARTENSH, . & RAYSSIGUÅ'YR., (1980) Magnesium metabolism andhypomagnesaemia. In: Digestive Physiology and Metabolism in Ruminants (Ruckebusch, Y. & Thivend, P., eds.), pp. 447-466, MTP Press, Lancaster, England.
64. O'CONNER,A. M. & BEEDE,D. K. (1986) Effects of lasalocid and monensin on in vitro apparent absorption of magnesium and sodium by duodenal tissue in Ussing chambers. /. Anim. Sci. 63 (Suppl. 1): 447 (abs.).
65. MOORE,R. J.,KORNEGAYE., T. & LINDEMANNM, . D. (1986) Effect of dietary oat hulls or wheat bran on mineral utilization in growing pigs fed diets with or without salinomycin. Can. J. Anim. Sci. 66: 267-276.
66. ZINN, R. A. (1986) Effect of salinomycin supplementation on characteristics of digestion and feedlot performance of cattle. /. Anim. Sci. 63: 1996-2004.
67. Knuc,D. J., GREENEL, .W., SCHELLINGG,. T. & BYERSF, .M. (1985) Effects of monensin on monovalent ion metabolism and tissue concentrations in lambs. /. Anim. Sci. 60: 1479-1484.
68. COSTA,N. D., GLEEDP, .T., SANSÃ"NB,.F., SYMONDSH, . W. & ALLEN, W. M. (1985) Monensin and narasin increase selenium and zinc absorption in steers. In: Trace Element Metabolism in Man and Animals (Mills, C. F., Bremner, I. & Chesters, J. K., eds.), pp. 472-474, Commonwealth Agricultural Bureaux, Slough, UK.
69. ANDERSONP, . H., BERRETTS, ., CATCHPOLE].,, GREGORYM, . W. & BROWN,D. C. (1983) Effect of monensin on the selenium status of sheep. Vet. Ree. 113: 498-500.
70. OLUMEYAND,. B., NAGARAJAT,. G., MILLERG, . W., FREY,R. A. & BOYER,J. E. (1986) Rumen microbial changes in cattle fed diets with or without salinomycin. Appi. Environ. Microbiol. 51:340-345.
71. IVANM, ., VEIRAD, . M. & KELLEHERC,. A. (1986) The alleviation of chronic copper toxicity in sheep by ciliate protozoa. Br. J. Nutr. 55:36-41.
72. SUTTLE,N. F. (1984) Effects of organic and inorganic sulfur on the availability of dietary copper to sheep. Br. f. Nutr. 32:559-569.
72. ELSASSERT,. H. (1984) Potential interactions of ionophore drugs with divalent cations and their function in the animal body. /. Anim. Sci. 59: 845-853.
73. LEE,D. B. N., BRAUTBARN,. & KLEEMANC,. R. (1981) Disorders of phosphorus metabolism. In: Disorders of Mineral Metabolism (Bronner, F. & Coburn, ]. W., eds.), vol. 3, pp. 283-421, Academic Press, New York, NY.
74. BAFUNDOK, . W., BAKERD, . H. & FITZGERALDP,. R. (1984) The iron-zinc interrelationship in the chick as influenced by Eimeria acervulina infection. /. Nutr. 114: 1306-1312.
Related topics
Authors:
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post





.jpg&w=3840&q=75)