Antimicrobial Residues and Resistance: Understanding and Managing Drug Usage on Dairy Farms
Published: October 11, 2013
By: Pamela Ruegg, DVM, MPVM, University of Wisconsin, Madison
Introduction
In modern dairy cattle operations, antimicrobials are administered for both therapeutic and prophylactic purposes. Most antimicrobials are used therapeutically but some antimicrobials are used to prevent disease in healthy animals during periods of increased susceptibility. Mastitis is one of the most frequent infectious diseases in dairy cattle and accounts for most of the doses of antibiotics given to dairy cows (Pol and Ruegg, 2006a). Lactating cows may be treated for clinical mastitis or to pursue bacteriological cure of a subclinical case. Antimicrobials are also used to treat other infectious diseases of dairy cows, including respiratory and uterine infections and infectious foot disease.
The use of antimicrobials to treat food animals has the potential to affect human health through 2 mechanisms: 1) increasing the risk of antimicrobial residues, and 2) influencing the generation or selection of antimicrobial resistant foodborne pathogens (Yan and Gilbert, 2004). The risk of antimicrobial residues is well known and has been addressed through the use of appropriate regulatory mechanisms but there is increasing concern about the impact of antimicrobial usage in food animals on the development of antimicrobial resistance. The objective of this paper is to review data about the relationship between antimicrobial resistance and the use of antimicrobials in dairy cattle with emphasis on antimicrobials used for treatment of mastitis.
Antimicrobial Concepts and Definitions
The terms antimicrobial and antibiotic are often used interchangeably but are not synonymous. In technical terms, "antibiotics" refer only to substances of microbial origin (such as penicillin) that are active against other microbes while "antimicrobial" refers to any substance (including synthetic compounds) which destroys microbes (Guardabasse and Courvalin, 2006). Antimicrobial agents interfere with specific bacterial processes needed for growth or division of cells. Compounds that inhibit bacterial growth are termed bacteriostatic while those that kill the bacteria are termed bactericidal. Antibacterial agents can be bacteriostatic when they reach the minimum inhibitory concentration (MIC) but become bactericidal when they reach a higher concentration, (the minimum bactericidal concentration (MBC)). If the MIC and the MBC are distinctly separated, the agent is considered bacteriostatic. If the MBC is close to the MIC, the compound is considered to be bactericidal (Prescott, 2000a).
Bacterial resistance can be intrinsic or acquired. An example of intrinsic resistance is a Gram-negative bacterium that has an outer membrane that is impermeable to the antibiotic. Acquired resistance occurs when a previously susceptible bacterium becomes resistant through mutation (vertical evolution) or acquisition of new DNA (horizontal evolution). Mutation is the result of a random event that occurs spontaneously. The presence of antibiotic will subsequently select for the resistant mutants (Prescott, 2000b).
Methods of horizontal evolution include conjugation, uptake of free DNA, and transduction. Transduction occurs when bacteriophages transfer genetic material from one bacterium to another. This process may involve genetic material that confers antibiotic resistance, like the transference of the β-lactamase gene from resistant to susceptible staphylococci (Prescott, 2000b). Mobile DNA segments (transposons) can transfer resistance among plasmids or to the genome. A specific transposon (integron) can accumulate resistance genes and may be a mechanism of development of multiple-antimicrobial resistance (Hall and Collis, 1995).
Several mechanisms of resistance have been described. Some bacteria contain enzymes that inactivate antibiotics. The most well known example is β-lactamase. These enzymes inactivate β-lactam antibiotics by cleaving the β-lactam rings. Some bacteria develop resistance by preventing the antibiotic from entering the bacterial cell or by increasing the removal of the drug out of the cell (faster than it enters the cell). Reduced ability to enter the cell occurs with resistance to fluoroquinolones, aminoglycosides and penicillins and can be overcome by increasing the drug concentration (Neu and Gootz, 2005, Levy, 2002). Increased removal of tetracycline is encoded by genes such as tet(A) and results in resistance of many enterobacteriaceae. Similar mechanisms have been described for resistance to erythromycin, chloramphenicol and ciprofloxacin (Levy, 2002). Structural changes in some bacteria can block activity of some antibiotics that successfully enter bacterial cells. For example, proteins responsible for cell wall synthesis of enterococci have low affinity for cephalosporins making these bacteria inherently resistant (Neu and Gootz, 2005). Elimination of the binding site by resistance genes carried on plasmids can result in resistance to macrolide, lincosamide, and streptogramin B (MLS resistance). Likewise, the tet(M) gene, produces a protein that can inhibit binding of tetracycline (Levy, 2002). Bacteria can also produce alternative binding sites that are resistant to inhibition. This mechanism is observed in methicillin resistant Staphylococcus aureus (MRSA), which produce the penicillin binding protein PBP2 encoded by mecA (Neu and Gootz, 2005).
Antimicrobial Susceptibility
Tests Antimicrobial susceptibility tests measure the ability of an antimicrobial agent to inhibit bacterial growth in vitro and are performed using methods that are based on either dilution or diffusion (Walker, 2000). The agar disc diffusion (ADD) is one of the most common methods and is referred to as the "Kirby-Bauer method." A standardized suspension of bacteria is streaked over a Muller-Hinton agar plate and antimicrobial impregnated discs are applied. During an overnight incubation, a gradient of antimicrobial concentration is established around the discs. The highest concentration is closest to the disc and progressively lower concentrations occur as distance from the disc increases. If the bacteria are susceptible to the antimicrobial tested, a distinct inhibition zone will be observed. If the bacteria are resistant to the antimicrobial, bacterial growth will be observed close to the antimicrobial disc. The diameter of each inhibition zone is recorded and the outcome is interpreted for each antimicrobial using standards based on the size of the zone of inhibition (Walker, 2000; Clinical and Laboratory Standards Institute, 2002). The ADD test is the most widely used method in veterinary medicine because it is inexpensive, does not require specialized equipment and is flexible enough to test different drugs. The major disadvantage is that results are qualitative, therefore the antimicrobial dose cannot be adjusted to maximize clinical outcome (Walker, 2000).
In dilution tests, microorganisms are tested for their ability to produce visible growth on a series of agar plates (agar dilution) or in broth (broth dilution) containing dilutions of the antimicrobial agent. These methods generate both quantitative and qualitative outcomes. Agar dilution is considered the "gold standard" but is costly and cumbersome (Walker, 2000). The use of broth microdilution is becoming more common in veterinary laboratories due to the development of automated systems. Serial dilutions of antimicrobials are inoculated with a standardized inoculum of bacteria and are incubated at 35° C for 16 to 20 hours. The MIC is considered to be the lowest concentration of antimicrobial agent that completely inhibits bacterial growth (Walker, 2000; Clinical and Laboratory Standards Institute, 2002). Like diffusion tests, outcomes of microdilution may be expressed as categorical outcomes (susceptible, intermediate, or resistant) based on breakpoints determined by the Clinical and Laboratory Standards Institute (CLSI) standards (Walker, 2000). The CLSI definition of susceptible implies "that there is a high likelihood of a favorable clinical outcome when the drug is administered at label dosage, because of adequate pharmacodynamic parameters relative to the MIC of the causative organism." (Clinical and Laboratory Standards Institute, 2002). The intermediate category is applicable to isolates that are "moderately susceptible" to an antibiotic that can be used for treatment at a higher dosage because of its low toxicity or because the antibiotic is concentrated at the focus of infection (e.g. urine). The resistant "category implies that there will not be a favorable clinical outcome, because the achievable systemic concentrations of the agent will be lower than the MIC of the causative organism with normal dosage schedules and/or fall in the range or where specific microbial resistance mechanisms are likely (e.g. β-lactamase), and clinical efficacy has not been reliable in treatment studies." (Clinical and Laboratory Standards Institute, 2002).
Interpretive criteria (breakpoints) for MIC or zone diameter values used to indicate whether the isolate is susceptible or resistant are determined by a national panel of experts (CLSI, formerly called the National Committee on Clinical Laboratory Standards or NCCLS) (Clinical and Laboratory Standards Institute, 2002). Only a few antimicrobials (penicillin/novobiocin, pirlimycin and ceftiofur) have a breakpoint for bovine mastitis. Other antimicrobials (ceftiofur, enrofloxacin, florfenicol, spectinomycin, and tilmicosin) have a veterinary breakpoint for bovine respiratory disease. Extrapolation of susceptibility criteria to other veterinary diseases (such as mastitis) may lead to incorrect prediction of clinical outcome. For example, in vitro susceptibility tests do not always predict clinical efficacy, especially for mastitis (Walker, 2000; Constable and Morin, 2003, Hoe and Ruegg, 2005). Breakpoints for pirlimycin are veterinary validated for mastitis but also appear to be poor predictors of treatment outcomes of mild and moderate clinical cases of mastitis (Hoe and Ruegg, 2005). Various factors may explain this issue. Antimicrobial susceptibility is tested in vitro, and laboratory conditions are different than the mammary gland environment (Walker, 2000). Proteins in milk may bind to antimicrobials therefore decreasing their activity (Fang and Pyorala, 1996). Finally, incomplete knowledge of the pharmacokinetics of intramammary compounds may lead to uncertain concentration of the antimicrobial at the site of infection (Sandholm et al., 1990).
Usage of Antimicrobials in Agriculture
The most comprehensive data about antimicrobial usage in the U.S. comes from the National Animal Health Monitoring System (NAHMS) (USDA, 2005). NAHMS estimated that more than half (55%) of the operations utilized medicated milk replacers (usually tetracyclines (+/- neomycin) or decoquinate). Chlortetracycline and sulfamethazine were the most commonly used antimicrobials in heifer rations (USDA, 2005). About 10% of calves were treated for pneumonia or diarrhea and one third of treated cases received florfenicol. Macrolides (16% of the treatments), β-lactams (not cephalosporins )(14%), and cephalosporins (14%) were also used. Calves with diarrhea received sulfonamides (24% of the treatments), tetracyclines (22%), β-lactams (not cephalosporins )(14%), aminoglycosides (11%) and cephalosporins (11%) (USDA, 2005). Weaned heifers were treated less frequently. Overall, 5% and 0.5% of all weaned heifers were treated for pneumonia and diarrhea, respectively. Tetracyclines were used for about one third of pneumonia treatments, florfenicol (26%) and macrolides (17%). Cephalosporins were used to treat more than half of the diarrhea cases, while β-lactams (not cephalosporins) (13%), tetracyclines (12%), and sulfonamides (11%) were used less frequently (USDA, 2005).
Foot infections in adult cows occurred in 52% of the dairy farms, while 50% and 42% of the producers reported the occurrence of pneumonia or metritis in adult cows, respectively (USDA, 2005). Most cases of pneumonia received antimicrobial treatment but only two thirds of the animals with metritis and foot infections received antimicrobials. The relatively low incidence of many diseases, resulted in a small proportion of the adult cows receiving antimicrobial treatments (< 14% of animals were treated for pneumonia, metritis or foot infections). Almost all (99%) of producers used antimicrobials to treat pneumonia (99%) and metritis or foot infections (90% each). Cephalosporins (67%), β-lactams (13%), and tetracyclines (12%) were used most frequently for treatments of pneumonia. Tetracyclines (41%), β-lactams (31%), and cephalosporins (23%) were used most frequently for treatments of metritis. Tetracyclines (42%), cephalosporins (30%), and β-lactams (17%) were used most frequently for treatments of foot infections.
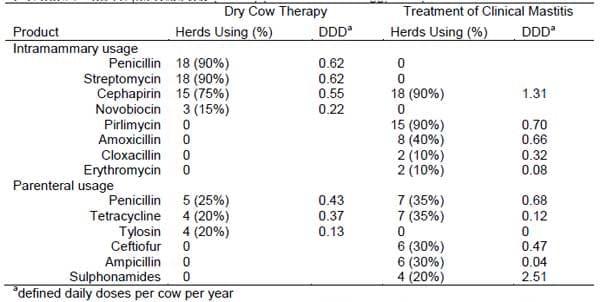
Pol and Ruegg (2006) recently developed a method to quantify antimicrobial usage and treatment practices. Data on disease prevalence and treatment practices of farms (n = 20) with bulk tank SCC >250,000 cells/ml were obtained during a farm visit and a standardized estimate of antimicrobial usage was developed using a Defined Daily Dose (DDD) . Density of antimicrobial usage was expressed as the number of DDD per adult cow per year. Farmers reported that penicillin was the compound most commonly used for dry cow therapy and cephapirin was most commonly used for treatment of clinical mastitis (Table 1). Additional antimicrobial exposures occurred for treatment of other diseases (foot infections, respiratory disease and metritis) and consisted of ceftiofur (0.59 DDD/cow/year; used on 17 farms), tetracycline (0.17 DDD/cow/year; 12 farms), penicillin (0.52 DDD/cow/year, used on 7 farms), ampicillin (0.07 DDD/cow/year, used on 8 farms) and sulphonamides (0.57 DDD/cow/year, used on 4 farms).
Table 1. Use of antimicrobials for treatment and prevention of mastitis on selected WI dairy farms with bulk tank SCC >250,000 cells/ml (n = 20) (from Pol and Ruegg, 2006)
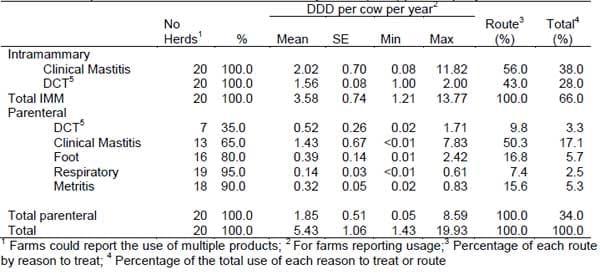
The estimated overall exposure to antimicrobials was 5.43 DDD/cow/year composed of 3.58 and 1.85 DDD of intramammary and parenteral antimicrobials, respectively (Table 2). Intramammary antimicrobials used for treatment of mastitis accounted for 56% of the intramammary usage and for 38% of the total usage. Parenteral antimicrobials used for treatment of mastitis accounted for about half of the parenteral usage and 17% of the total usage. About 80% of all antimicrobials were used for treatment or prevention of mastitis (DCT, 28%; intramammary compounds for clinical mastitis, 38%; parenteral compound for clinical mastitis, 17%).
Table 2. Descriptive statistics of estimated Defined Daily Doses (DDD) per cow per year
Eleven producers (of 20) reported extra-label use of antimicrobials via an intramammary route. Ampicillin was used for intramammary treatments on 6 farms, ceftiofur and gentamycin were used for intramammary treatments on 3 farms each. Two farmers reported the extra-label use of penicillin, 1 producer reported the extra-label use of miconazole, and 1 producer used extra-label oxytetracycline for IMM treatments. Compounds prepared by veterinarians with unknown ingredients were used in 2 farms. Two producers reported use of a prohibited compound for IMM treatments (sulfamethoxazole/trimethoprim).
Considerable among farm and region variability has been reported for mastitis therapeutics (Zwald et al., 2004; Sato et al., 2005; Kirk et al., 2005). Almost 80% of the producers (n = 99) surveyed by Zwald et al. (2004) reported the parenteral use of antimicrobials to treat some cases of clinical mastitis but in a smaller study (n = 30) Sato et al. (2005) found that < one third of WI producers utilized systemic antimicrobials for mastitis treatments. About 40% and 60% of the California producers reported the usage of injectable antimicrobials to treat mild and severe cases of mastitis, respectively (Kirk et al., 2005). Sato et al. (2005) reported that only 60% of the conventional farms regularly used intramammary tubes to treat clinical mastitis but his estimates contrasts with the 93% reported in NAHMS survey of dairy cattle (USDA, 1996).
Usage of Antimicrobials and Susceptibility
Methodological and reporting differences among studies complicate the ability to determine if resistance to antimicrobials is emerging in mastitis pathogens. However, similar retrospective studies have reported comparable findings (Erskine et al, 2002; Makovec and Ruegg, 2003). Erskine et al. (2002) reported the trends in antimicrobial susceptibility of about 2,800 mastitis isolates obtained from samples submitted by veterinarians to a central laboratory during 7 years. Antimicrobial susceptibility was tested using ADD. The proportion of susceptible isolates did not change during the 7-yr period for most of bacteria-antimicrobial combinations. Makovec and Ruegg (2003) reported similar conclusions in a larger study (8,900 samples), where a reduced resistance to β-lactams antimicrobials was observed in S. aureus, CNS, and Strep. spp.
Several studies have described differences in susceptibility of isolates obtained from farms with different histories of potential exposure to antimicrobials (Tikofsky et al., 2003; Sato et al., 2004; Rajala-Schultz et al., 2004; Berghash et al., 1983). Tikofsky et al., (2003) used ADD to study antimicrobial susceptibility of S. aureus isolated from cows on organic (n = 144) and conventional herds (n = 117). Significantly fewer isolates obtained from conventional herds were susceptible to ampicillin, penicillin or tetracycline. However, antimicrobial usage was not quantified. Sato et al. (2004) studied S. aureus isolated from bulk milk obtained from organic (Wisconsin n = 179; Denmark n = 75) and conventional (Wisconsin n = 152; Denmark n = 7) herds in Wisconsin and Denmark. Isolates with MIC higher than MIC90 (MIC needed to inhibit 90% of isolates) were classified as isolates with reduced susceptibility. Isolates with a MIC lower than MIC90 were termed as isolates with high susceptibility. The quantity of antimicrobial used by conventional farmers was unknown. The probability of having a resistant isolates in conventional herds was higher only for ciprofloxacin in Wisconsin and for avilamycin in Denmark. Significant differences were found in the susceptibility pattern among countries. Wisconsin isolates had a higher probability of reduced susceptibility (defined as MIC > MIC90) for bacitracin, gentamycin, kanamycin, penicillin, sulphametoxazole, tetracycline and trimetroprim.
Rajala-Schultz et al. (2004) studied the antimicrobial susceptibility of CNS isolated from primiparous and multiparous cows using a microbroth dilution system. A total of 139 isolates were studied but the number of isolates by parity was not reported. The antimicrobial usage pattern in the single herd studied was not evaluated quantitatively. Penicillin, ceftiofur, cephapirin and dihydrostreptomycin were the drugs used more frequently. The authors hypothesized that first lactation cows might be exposed to a lower level of antimicrobial exposure due to differences in antimicrobial therapies among the two groups. The study failed to detect significant differences among primiparous and multiparous cows. However, a trend was observed in the differences of the proportion of CNS isolates resistant to penicillin.
One early study attempted to compare herds with exposures to different amounts of antimicrobials (Berghash et al., 1983). In that study, antimicrobial susceptibility of Strep. agalactiae, Strep. spp, and S. aureus was studied using agar dilution. Herds were classified as either high antibiotic-use or low antibiotic-use . The "high antibiotic-use" group used comprehensive DCT with cephapirin. The "low antibiotic-use" group used IMM antimicrobials only for treatment of clinical cases of mastitis. Isolates of Strep. agalactiae obtained "high antibiotic-use" (n = 53) presented high levels of resistance to β-lactams, tetracyclines, aminoglycosides and novobiocin as compared to isolates obtained "low antibiotic use" herds (n = 49). No differences were found in the antimicrobial susceptibility of S. aureus isolates or Strep app. among groups.
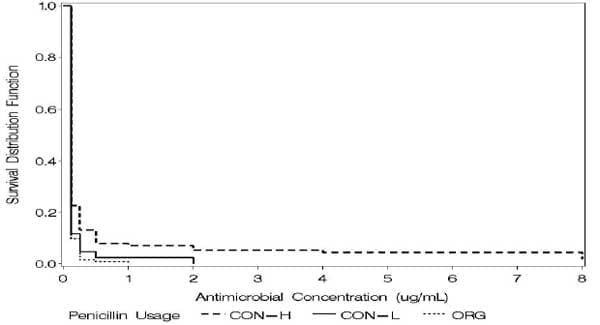
Pol and Ruegg (2006b) recently analyzed relationships between usage of antimicrobials on dairy farms and results of antimicrobial susceptibility testing of mastitis pathogens. Exposure to selected antimicrobials (n = 10) was standardized using DDD. Farms (n = 40) were categorized based on levels of antimicrobial exposure: Organic (no usage); Conventional - low usage, Conventional - high usage. The MIC of selected antimicrobials was determined using a microbroth dilution system for isolates of Staphylococcus aureus (n = 137), coagulase negative Staphylococci (n = 294) and Strep. spp. (n = 95) obtained from subclinical mastitis infections. Most isolates were inhibited at the lowest dilution tested of most antimicrobials. For Staph. aureus and CNS, the MIC was associated with level of exposure to penicillin and pirlimycin (Fig 1). For CNS, farm type was also associated with the MIC of ampicillin, and tetracycline. For Strep spp., farm type was associated with MIC of pirlimycin and tetracycline. For all mastitis pathogens studied, the MIC of pirlimycin increased with increasing exposure to defined daily doses of pirlimycin. The level of exposure to most other antimicrobials was not associated with minimum inhibitory concentration of mastitis pathogens. Usage of two compounds commonly administered for treatment of intramammary infections (penicillin and pirlimycin) was associated with resistance of mastitis pathogens but usage of many other commonly used compounds was not. A dose-response effect between pirlimycin usage groups and pirlimycin MIC was observed for all isolates studied. The usage of penicillin was associated with reduced susceptibility of Staph. aureus (data not shown) and CNS isolates (Fig 1). However, the usage of cephapirin (a widely used antimicrobial for intramammary infections treatments) was not associated with reduced susceptibility of any of the studied pathogens.
Figure 1. Kaplan-Meier plot showing survival proportion of CNS on organic farms , no use of penicillin or use ≤ than the first quartile of usage (CON-L) or use > than the first quartile of usage (CON-H).
Conclusions
Society will continue to be concerned about the development and transfer of antimicrobial resistance from animal agriculture and veterinarians will need to be responsive to their concerns. Antimicrobial usage is difficult to assess on dairy farms but recent studies indicate that many dairy cows are exposed to >5 DDD/cow/year. The majority of antimicrobial usage on dairy farms is related to treatment and prevention of mastitis. The amount of exposure to some antimicrobials has been linked to increased resistance but exposure to other commonly used antimicrobials has not. While exposure to antimicrobial has been linked to resistance, there is no current evidence that resistance is increasing. Further studies should be designed to evaluate the temporal relationship of exposure and resistance and to determine optimal usage patterns of antimicrobials on dairy farms.
References
Berghash S.R., J.N. Davidson, J.C. Armstrong, and G. M. Dunny. 1983. Effects of antibiotic treatment of nonlactating dairy cows on antibiotic resistance patterns of bovine mastitis pathogens. Antimicrobial Agents And Chemotherapy, Nov. p. 771-776
Constable, P.D., Morin, D.E. 2003. Treatment of clinical mastitis using antimicrobial susceptibility profiles for treatment decisions. Vet Clin Food Anim 19: 139 – 155.
Erskine, R.J., R.D. Walker, C.A. Bolin, B.C. Bartlett, and D.G.White. 2002. Trends in antibacterial susceptibility of mastitis pathogens during a seven-year period. J. Dairy Sci. 85:1111–1118.
Fang, W., Pyorala, S. 1996. Mastitis-Causing Escherichia coli: Serum Sensitivity and Susceptibility to Selected Antibacterials in Milk. J. Dairy Sci. 1996 79: 76-82.
Food and Drug Administration – Center for Veterinary Medicine (FDA- CVM). 2005. Green Book Online: http://www.fda.gov/cvm/greenbook.html
Guardabassi, L. and P. Courvalin. 2006. Modes of antimicrobial action and mechanisms of bacterial resistance. Chap 1. in Aarestrup, F. M., Antimicrobial resistance in bacteria of animal origin. ASM Press, Washington DC.
Hall, R., Collis, C. 1995. Mobile gene cassettes and integrons: capture and spread of genes by site-specific recombination. Mol. Microbiol. 15: 593-600.
Hoe, F.G.H and P.L. Ruegg. 2005a. Relationship between antimicrobial susceptibility of clinical mastitis pathogens and treatment outcome in cows. JAVMA 227 (9) : 1461-8
Jones, FT, Ricke, SC. 2003. Observations on the History of the Development of Antimicrobials and Their Use in Poultry Feeds. Poultry Science. 82:613 – 617.
Kirk J. H., B. McCowan, E. R. Atwill, K. S. Glenn, G. E. Higginbotham, C. A. Collar, A. Castillo, B. A.
Reed, N. G. Peterson, J. S. Cullor. 2005. Association of minimum inhibitory concentration cluster patterns with dairy management practices for environmental bacteria isolated from bulk tank milk. J Dairy Sci. 88(10):3710-20.
Levy, S.. 2002. The Antibiotic Paradox 2nd Edition. Perseus Publishing 15-56.
Neu, H., Gootz T. Antimicrobial chemotherapy. In http://gsbs.utmb.edu/microbook/toc.htm
Makovec J. A. and P.L. Ruegg. 2003. Antimicrobial resistance of bacteria isolated from dairy cow milk samples submitted for bacterial culture: 8,905 samples (1994–2001). J. Am. Vet. Med. Assoc. 222 (11): 1582-9
Pol, M. and P. L. Ruegg. 2006a. Treatment practices and quantification of antimicrobial drug usage in conventional and organic dairy farms in Wisconsin J. Dairy Sci. 89:in press.
Pol, M. and P. L. Ruegg. 2006b. Relationship between reported antimicrobial usage and antimicrobial susceptibility of Gram-positive mastitis pathogens. J. Dairy Sci. 89:in press.
Prescott J.F. Antimicrobial drug action and interaction: an introduction. 2000 a. In: Antimicrobial Therapy in Veterinary Medicine, J.F. Prescott, J.D. Baggott, and R.D. Walker, eds. 3rd edition, Iowa State University Press, Ames, Iowa. pp 3-11.
Prescott J.F. Antimicrobial drug resistance and its epidemiology. 2000 b. In: Antimicrobial Therapy in Veterinary Medicine, J.F. Prescott, J.D. Baggott, and R.D.
Rajala-Schultz P.J., K.L. Smith, J.S. Hogan and B.C. Love, Antimicrobial susceptibility of mastitis pathogens from first lactation and older cows, Vet. Microbiol. 102 (2004), pp. 33–42
Sato K, Bennedsgaard TW, Bartlett PC, Erskine RJ, Kaneene JB. 2004. Comparison of antimicrobial susceptibility of Staphylococcus aureus isolated from bulk tank milk in organic and conventional dairy herds in the Midwestern United States and Denmark. J Food Prot. 67(6): 1104-10.
Sato K.; Bartlett, PC; Erskine RJ and Kaneene, JB. 2005. A comparison of production and management between Wisconsin organic and conventional dairy herds. Livestock Production Science Volume 93, Issue 2 , 15 April 2005, Pages 105-115
Sawant, A. A., Sordillo, L. M., Jayarao, B. M. 2005. A Survey on Antibiotic Usage in Dairy Herds in Pennsylvania J. Dairy Sci. 88: 2991-2999
Tikofsky, L.L., J.W. Barlow, C. Santisteban, and Y. H. Schukken. 2003. A comparison of antimicrobial susceptibility patterns for staphylococcus aureus in organic and conventional dairy herds. Microbial Drug Resistance 9:supplement 1, 39-45
Walker, eds. 3rd edition, Iowa State University Press, Ames, Iowa. pp 27-49.
Sandholm M, Kaartinen L, Pyorala S. 1990. Bovine mastitis--why does antibiotic therapy not always work? An overview. J Vet Pharmacol Ther. 13(3):248-60.
Walker R.D. Antimicrobial susceptibility testing and interpretation of results. 2000. In: Antimicrobial Therapy in Veterinary Medicine, J.F. Prescott, J.D.
Yan S.S., J. M. Gilbert. 2004. Antimicrobial drug delivery in food animals and microbial food safety concerns: an overview of in vitro and in vivo factors potentially affecting the animal gut microflora. Adv. Drug Deliv. Rev. 56 , pp. 1497–1521.
Zwald AG, Ruegg PL, Kaneene JB, Warnick LD, Wells SJ, Fossler C, Halbert LW. 2004. Management practices and reported antimicrobial usage on conventional and organic dairy farms. J Dairy Sci. 87(1):191-201.
This paper was presented at Central Veterinary Conference, Kansas City, MO, August 2013 and published on the Milk Quality, University of Wisconsin´s website. http://milkquality.wisc.edu/. Engormix.com thanks for this huge contribution.
Related topics
Authors:
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post11 de febrero de 2014
A very informative paper which most producers should read, if not to understand the methodology of the antibiotic (bacteriostatic and bactericide) and the evolution of bacterial resistance to antibiotics.
We process 3500 litres of milk daily and are very cautious about atibiotic residues in our milk products,especially in the case of new generation antibiotics, where the inability of existing commercial test kits to detect certain antibiotic residues is a concern.
It is easy to say one should reduce the use of antibiotics, but in the case of lameness, enteritis, pneumonia, and mastitis, under estimating the health problem can be a dead or damaged animal, which raises questions on the economics and welfare of a treatment schedule. Obviously prevention is the preferred method, and as dairy farms develop and and incorporate modern housing, milking equipment, and adopt husbandry practises which reduce infection, this may be possible. However there still remains constraints in the form of climate, environment, individual animal susceptibility, and established management practises which may be more difficult to control. One fundamental objective is certainly prevention, followed if need be by more cautious application of antibiotics, completion of treatment schedules as recommended by supplier, and to follow an established health protocol for a treatment, instead of haphazard un recorded applications of single or multiple antibiotics.
10 de febrero de 2014
Would this situation be improved by the use of vaccines aimed at various mastitis causing organisms, such as the Hipra vaccine Startvac? Could enhancing the natural immune response of the cow reduce case numbers, and improve response to therapy, reducing overall antimicrobial use?
10 de febrero de 2014
Congratulations to Dr pamela for drying attention to this acute problem our future generation has to face ..Indiscriminate use of antibiotics and other antimicrobial medicines in livestock mostly use as food. since there is no recording system in public health therefore it is very difficult to understand its total impact on human health.I hope this paper will b taken seriously and more scientists will focus their attention towards this concern which will help in creating awareness against this future menance








.jpg&w=3840&q=75)