The use of algae in fish feeds as alternatives to fishmeal
Published: October 10, 2013
By: Eric C. Henry PhD, Research Scientist, Reed Mariculture Inc., USA
Fishmeal is very extensively used in feeds for fish as well as other animals. A recent global survey estimated aquaculture consumption of fishmeal at 3724 thousand tonnes in 2006 (Tacon and Metian 2008). Now it is becoming increasingly evident that such continued exploitation of this natural resource will ultimately become both environmentally and economically unsustainable.
Any satisfactory alternative feed ingredients must be able to supply comparable nutritional value at competitive cost. Conventional land-based crops, especially grains and oilseeds, have been favoured alternatives due to their low costs, and have proved successful for some applications when they were used as substitutes for a portion of the fishmeal.But even when these plant-based substitutes can support good growth they can cause significant changes in the nutritionalquality of the fish produced.
Why algae?
The reader may wonder why algae, including both macroalgae (‘seaweeds’) and microalgae (e.g. phytoplankton), and which are popularly thought of as ‘plants’, would be good candidates to serve as alternatives to fishmeal in fish feeds. One fundamental consideration is that algae are the base of the aquatic food chains that produce the food resources that fish are adapted to consume. But often it is not appreciated that the biochemical diversity among different algae can be vastly greater than among land plants, even when ‘Blue-Green Algae’ (e.g. Spirulina), more properly called Cyanobacteria, are excluded from consideration. This reflects the very early evolutionary divergence of different algal groups in the history of life on earth. Only one of the many algal groups, the Green Algae, produced a line of descent that eventually gave rise to all the land plants.Therefore it can be difficult to make meaningful generalisations about the nutritional value of this extremely diverse group of organisms; rather it is necessary to consider the particular qualities of specific algae.
Protein and amino acids
Fishmeal is so widely used in feeds largely thanks to its substantial content of high-quality proteins, containing all the essential amino acids. A critical shortcoming of the crop plant proteins commonly used in fish feeds is that they are deficient in certain amino acids such as lysine, methionine, threonine, and tryptophan (Li et al. 2009), whereas analyses of the amino acid content of numerous algae have found that although there is significant variation, they generally contain all the essential amino acids. For example, surveys of 19 tropical seaweeds (Lourenço et al. 2002) and 34
edible seaweed products (Dawczynski et al. 2007) found that all species analysed containedall the essential amino acids, and these findings are consistent with other seaweed analyses (Rosell andSrivastava 1985, Wong and Peter 2000, Ortiz et al. 2006).
Analyses of microalgae have found similar high contents of essential amino acids, as exemplified by a comprehensive study of 40 species of microalgae from seven algal classes that found that, “All species had similar amino acid composition, and were rich in the essential amino acids” (Brown et al. 1997).
Taurine
One often-overlooked nutrient is the non-protein sulphonic acid taurine, which is sometimes lumped with amino acids in discussions of nutrition.Taurine is usually an essential nutrient for carnivorous animals, including some fish, but it is not found in any land plants. However, although taurine has been much less often investigated than amino acids, it has been reported in significant quantities in macroalgae such as Laminaria, Undaria, and Porphyra (Dawczynski et al. 2007, Murata and Nakazoe 2001) as well as certain microalgae, for example the green flagellate Tetraselmis (Al-Amoudia and Flynn 1989), the red unicellular alga Porphyridium (Flynn and Flynn 1992), the dinoflagellate Oxyrrhis (Flynn and Fielder 1989), and the diatom Nitzschia (Jackson et al. 1992).
Pigments
A few algae are used as sources of pigments in fish feeds. Haematococcus is used to produce astaxanthin, which is responsible for the pink colour of the flesh of salmon. Spirulina is used as a source of other carotenoids that fishes such as ornamental koi can convert to astaxanthin and other brightly coloured pigments. Dunaliella produces large amounts of beta-carotene.
Lipids
In addition to its high content of high-quality protein, fishmeal provides lipids rich in ‘PUFAs’, or polyunsaturated omega-3 and omega-6 fatty acids. These are the ‘fish oil’ lipids that have become highly prized for their contribution to good cardiovascular health in humans. But it is not always appreciated that algae at the base of the aquatic food chain in fact originate these ‘fish oil’ fatty acids.These desirable algal fatty acids are passed up the food chain to fish, and they are indeed essential nutrients for many fish.
Algae have been recognised as an obvious alternative source of these ‘fish oil’ fatty acids for use in fish feeds (Miller et al. 2008), especially eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and arachidonic acid (ARA). There is a substantial literature devoted to analysis of the PUFA content of microalgae, particularly those used in aquaculture, because they have long been recognised as the best source of these essential nutrients for production of zooplankton necessary for the first feeding of larval fish, as well as filter-feeding shellfish.
Many shellfish producers are aware the sterol profile of feed lipids is of critical importance, but much less attention has been paid to the importance of the sterol profile of fish feeds. Aside from alterations in the normal sterol profile of the fish, the possible endocrine effects of plant phytosterols in fish feeds (e.g. soy phytohormones) have yet to be thoroughly investigated (Pickova and Mørkøre 2007).
Use of algae in aquaculture
Many different algae already play a vital role in aquaculture. It is widely known that the addition of microalgae to larval fish culture tanks confers a number of benefits, such as preventing bumping against the walls of the tanks (Battaglene and Cobcroft 2007), enhancing predation on zooplankton (Rocha et al. 2008), enhancing the nutritional value of zooplankton (Van Der Meeren et al. 2007), as well as improving larval digestive (Cahu et al. 1998) and immune (Spolaorea et al. 2006) functions.
Furthermore, it has also been shown that larvae of some fishes benefit greatly by direct ingestion of microalgae (Reitan et al. 1997). One study has even shown that that live zooplankton could be eliminated from the larval diet of Red Drum if microalgae were fed along with a formulated microparticulate diet (Lazo et al.).It is not surprising that the biochemical compositions of certain marine microalgae are well-matched to the nutritional requirements some marine fish. Larval feeds are probably deserving of the most attention in efforts to discover how algae can best be used in fish feeds, because microalgae are a natural component of the diet of many larval fish, either consumed directly or acquired from the gut contents of prey species such as rotifers and copepods. Existing protocols that use microalgae to improve the PUFA profile of live prey (Table 1) demonstrate how effectively an algal feed can enhance the nutritional value of these live feeds.
Table 1. Nutritional profiles of rotifers enriched using optimized protocols based on culture using Reed Mariculture RotiGrow Plus® and enriched with N-Rich® feeds
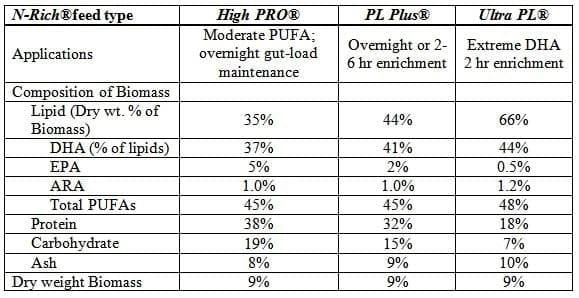
Use of algae in formulated fish feeds
Various species of macroalgae and microalgae have been incorporated into fish feed formulations to assess their nutritional value, and many have been shown to be beneficial: Chlorella or Scenedesmus fed to Tilapia (Tartiel et al. 2008); Chlorella fed to Korean rockfish (Bai et al. 2001); Undaria or Ascophyllum fed to Sea Bream (Yone et al. 1986); Ascophyllum, Porphyra, Spirulina, or Ulva fed to Sea Bream (Mustafa and Nakagawa 1995); Gracilaria or Ulva fed to European Sea Bass (Valente et al. 2006); Ulva fed to Striped Mullet (Wassef et al. 2001); Ulva or Pterocladia fed to Gilthead Sea Bream (Wassef et al. 2005);Porphyra, or a Nannochloropsis-Isochrysis combination fed to Atlantic Cod (Walker et al. 2009, 2010). Unfortunately, it has rarely been possible to determine the particular nutritional factors responsible for these beneficial effects, either because no attempt was made to do so, or poor design of the study.
For example, in one of the few studies that has focused on the effects of substituting algal protein for gluten protein, the control and all the test diets contained casein plus added methionine and lysine, no analysis of the algal protein was provided, and the algal protein (a biofuel process by-product) contained very high levels of aluminium and iron (Hussein et al. 2012). More and better-designed studies are necessary before we will have a good understanding of how algae can best be used in fish feeds.
Choosing the right algae
Often the algae chosen for fish feeding studies appear to have been selected largely for convenience, because they are low-cost and commercially available. For example, microalgae such as Spirulina,Chlorella and Dunaliella can be produced by low-cost open-pond technologies and are marketed as dry powders, and their nutritional profiles are well-documented. Macroalgae such as the ‘kelps’Laminaria, Undaria, and Durvillea, and the brown rockweed Ascophyllum, occur in dense stands that can be harvested economically, and they have a long history of use as sources of iodine, as soil amendments, and animal feed additives to supply trace elements.
In recent years there has been great interest in the potential of algae as a biofuel feedstock, and it has often been proposed that the protein portion remaining after lipid extraction might be a useful input for animal feeds (e.g. Chen et al. 2010). However, the algae chosen for biofuel production may not be optimal for use as a feed input, and the economic pressure for the lowest-cost methods of fuel production is likely to result in protein residues with contamination that makes them unfit for use as feed (e.g. Hussein et al. 2012).
By contrast, the high-value microalgae that are used in shellfish and finfish hatcheries are generally produced in closed culture systems to exclude contaminating organisms, and they cannot be dried before use without adversely affecting their nutritional and physical properties, greatly reducing their value as feeds. Inevitably their production costs are higher, but their exceptional nutritional value justifies the extra expense. Table 2 presents typical nutritional profiles of algae produced by Reed Mariculture Inc.
Table 2. Because these algae are produced using continuous-harvest technology that maintains exponential growth, their protein and lipid contents are comparable to those provided by fish feeds.
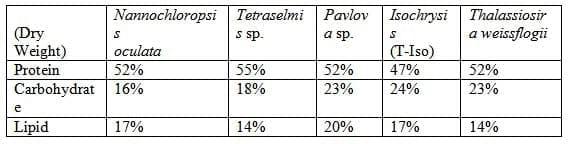 Just as it would be senseless to arbitrarily substitute one conventional crop plant for another (e.g. potatoes for soybeans) when formulating a feed, the particular attributes of each alga must be carefully considered. In addition to the protein/amino acid profile, lipid/PUFA/sterol profile, and pigment content, there are important additional considerations.
Just as it would be senseless to arbitrarily substitute one conventional crop plant for another (e.g. potatoes for soybeans) when formulating a feed, the particular attributes of each alga must be carefully considered. In addition to the protein/amino acid profile, lipid/PUFA/sterol profile, and pigment content, there are important additional considerations.The type and quantity of extracellular polysaccharides, which are very abundant in certain algae, can interfere with nutrient absorption, or conversely be useful binding agents in forming feed pellets. The thick cell walls of microalgae such as Chlorella can prevent absorption of the nutritional value of the cell contents. Inhibitory compounds such as the phenolics produced by some kelps, and brominated compounds produced by red algae such as Laurencia, can render an alga with an excellent nutritional analysis unsuitable for use in a feed. Depending on growth and processing conditions, algae can contain high concentrations of trace elements that may be detrimental.
Further careful study of the properties of numerous algae will be necessary in order to optimally exploit the great potential offered by this diverse group of organisms. But it is already apparent that algae will play an important part in the effort to move the formulation of fish feed “down the food chain” to a more sustainable future.
References
Al-Amoudia O.A., Flynn K.J. (1989). Effect of nitrate-N incorporation on the composition of the intracellular amino acid pool of N-deprived Tetraselmis marina. British Phycological Journal 24, 53-61
Bai S.C., Koo J.-W., Kim K.W., Kim S.K. (2001). Effects of Chlorella powder as a feed additive on growth performance in juvenile Korean rockfish, Sebastes schlegeli (Hilgendorf). Aquaculture Research 32, Issue Supplement s1, 92–98
Battaglene S.C., Cobcroft J.M. (2007). Advances in the culture of striped trumpeter larvae: A review. Aquaculture 268, 195–208
Brown M.R., Jeffrey S.W., Dunstan G.A. (1997). Nutritional properties of microalgae for mariculture. Aquaculture 151, 315–331
Cahu C.L., Zambonino Infante J.L., Péres A., Quazuguel P., Le Gall M.M. (1998). Algal addition in sea bass (Dicentrarchus labrax) larvae rearing: effect on digestive enzymes. Aquaculture 161, 479-489
Chen S., Chi Z., O’Fallon J.V., Zheng Y., Chakraborty M., Laskar D.D. (2010). System integration for producing microalgae as biofuel feedstock. Biofuels 1, 889-910
Dawczynski C., Schubert R., Jahreis G. (2007). Aminoacids, fatty acids, and dietary fibre in edible seaweed products. Food Chemistry 103, 891–899
Flynn K.J., Fielder J. (1989). Changes in intracellular and extracellular amino acids during the predation of the chlorophyte Dunaliella primolecta by the heterotrophic dinoflagellate Oxyrrhis marina and the use of the glutamine/glutamate ratio as an indicator of nutrient status in mixed populations. Marine ecology progress series. 53, 117-127
Flynn K.J., Flynn K. (1992). Non-protein free amines in microalgae: Consequences for the measurement of intracellular amino acids and of the glutamine/glutamate ratio. Marine ecology progress series 89, 73-79
Hussein E.E.-S., Dabrowski K., El-Saidy D.M.S.D., Lee B.-J. (2012). Enhancing the growth of Nile tilapia larvae/juveniles by replacing plant (gluten) protein with algae protein. Aquaculture Research published online: 5 MAR 2012 DOI: 10.1111/j.1365-2109.2012.03100.x
Jackson A.E., Ayer S.W., Laycock M.V. (1992). The effect of salinity on growth and amino acid composition in the marine diatom Nitzschia pungens. Canadian Journal of Botany 70, 2198-2201
Lazo J.P., Dinis M.T., Holt G.J., Faula C., Arnold C.R. (2000). Co-feeding microparticulate diets with algae: toward eliminating the need of zooplankton at first feeding in larval red drum (Sciaenops ocellatus). Aquaculture 188, 339-3512
Li P., Mai K, Trushenski J., Wu G. (2009). New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37, 43-53
Lourenço S.O., Barbarino E., De-Paula J.C., da S. Pereira L.O., Lanfer Marquez U.M. (2002). Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycological Research 50, 233–241
Miller M.R., Nichols P.D., Carter C.G. (2008). n-3 Oil sources for use in aquaculture – alternatives to the unsustainable harvest of wild fish. Nutrition Research Reviews 21, 85-96
Murata M., Nakazoe J. (2001). Production and use of marine algae in Japan. Japan Agricultural Research Quarterly 35, 281-290
Mustafa M.G., Wakamatsu S., Takeda T. (1995). Effect of algae as a feed additive on growth performance in red sea bream, Pagrus major. Trace Nutrients Research 12, 67–72
Pickova J., Mørkøre T. (2007). Alternate oils in fish feeds. European Journal of Lipid Science and Technology 109, 256–263
Ortiz J., Romero N., Robert P., Araya J., Lopez-Hernández J., Bozzo C., Navarrete E., Osorio A., Rios A. (2006). Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chemistry 99, 98–104
Rocha R.J., Ribeiro L., Costa R., Dinis M.T. (2008). Does the presence of microalgae influence fish larvae prey capture? Aquaculture Research 39, 362 – 369
Rosell K.-G., Srivastava L.M. (1985). Seasonal variations in total nitrogen, carbon and amino acids in Macrocystis integrifolia and Nereocystis luetkeana (Phaeophyta). Journal of Phycology 21, 304–309
Spolaorea P., Joannis-Cassan C., Duran E., Isambert A. (2006). Commercial applications of microalgae. Journal of Bioscience and Bioengineering 101, 87–96
Tacon A.G.J., Metian M. (2008). Global overview on the use of fish meal and fishoil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 285, 146–158
Tartiel M.B., Ibrahim E.M., Zeinhom M.M. (2008). Partial replacement of fish meal with dried microalga (Chlorella spp and Scenedesmus spp) in Nile Tilapia (Oreochromis niloticus) diets. 8th International Symposium on Tilapia in Aquaculture, pp. 801-811
Valente L.M.P., Gouveia A., Rema P., Matos J., Gomes E.F., Pinto I.S. (2006). Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 252, 85–91
Van Der Meeren T., Mangor-Jensen A., Pickova J. (2007). The effect of green water and light intensity on survival, growth and lipid composition in Atlantic cod (Gadus morhua) during intensive larval rearing. Aquaculture 265, 206-2173
Walker A.B., Fournier H. R., Neefus C.D., Nardi G.C., Berlinsky D.L. (2009). Partial replacement of Fish Meal with Laver Porphyra spp. in diets for Atlantic Cod. North American Journal of Aquaculture 71, 39-45
Walker A.B., Sidor I.F., O'Keefe T., Cremer M., Berlinsky D.L. (2010). Effects of replacement of fish meal protein by microalgae on growth, feed intake, and body composition of Atlantic Cod. North American Journal of Aquaculture 72, 343-353
Wassef E.A., El Masry M.H., Mikhail F.R. (2001). Growth enhancement and muscle structure of striped mullet, Mugil cephalus L., fingerlings by feeding algal meal-based diets. Aquaculture Research 32, 315–322
Wassef E.A., El-Sayed A.F.M., Kandeel K.M., Sakr E.M. (2005). Evaluation of Pterocladia (Rhodophyta) and Ulva (Chlorophyta) Meals as additives to Gilthead Seabream Sparus Aurata diets. Egyptian Journal Of Aquatic Research 31, Special Issue, 321-332
Wong K.H., Peter C.K. (2000). Nutritional evaluation of some subtropical red and green seaweeds: Part I — proximate composition, amino acid profiles and some physico-chemical properties. Food Chemistry 71, 475–482
Yone Y., Furuichi M., Urano K. (1986). Effects of dietary wakame Undaria penatifida and Ascophyllum nodosum supplements on growth, feed efficiency, and proximate compositions of liver and muscle of red sea bream [Chrysophrys major]. Bulletin of the Japanese Society of Scientific Fisheries 52, 1465-1468
Al-Amoudia O.A., Flynn K.J. (1989). Effect of nitrate-N incorporation on the composition of the intracellular amino acid pool of N-deprived Tetraselmis marina. British Phycological Journal 24, 53-61
Bai S.C., Koo J.-W., Kim K.W., Kim S.K. (2001). Effects of Chlorella powder as a feed additive on growth performance in juvenile Korean rockfish, Sebastes schlegeli (Hilgendorf). Aquaculture Research 32, Issue Supplement s1, 92–98
Battaglene S.C., Cobcroft J.M. (2007). Advances in the culture of striped trumpeter larvae: A review. Aquaculture 268, 195–208
Brown M.R., Jeffrey S.W., Dunstan G.A. (1997). Nutritional properties of microalgae for mariculture. Aquaculture 151, 315–331
Cahu C.L., Zambonino Infante J.L., Péres A., Quazuguel P., Le Gall M.M. (1998). Algal addition in sea bass (Dicentrarchus labrax) larvae rearing: effect on digestive enzymes. Aquaculture 161, 479-489
Chen S., Chi Z., O’Fallon J.V., Zheng Y., Chakraborty M., Laskar D.D. (2010). System integration for producing microalgae as biofuel feedstock. Biofuels 1, 889-910
Dawczynski C., Schubert R., Jahreis G. (2007). Aminoacids, fatty acids, and dietary fibre in edible seaweed products. Food Chemistry 103, 891–899
Flynn K.J., Fielder J. (1989). Changes in intracellular and extracellular amino acids during the predation of the chlorophyte Dunaliella primolecta by the heterotrophic dinoflagellate Oxyrrhis marina and the use of the glutamine/glutamate ratio as an indicator of nutrient status in mixed populations. Marine ecology progress series. 53, 117-127
Flynn K.J., Flynn K. (1992). Non-protein free amines in microalgae: Consequences for the measurement of intracellular amino acids and of the glutamine/glutamate ratio. Marine ecology progress series 89, 73-79
Hussein E.E.-S., Dabrowski K., El-Saidy D.M.S.D., Lee B.-J. (2012). Enhancing the growth of Nile tilapia larvae/juveniles by replacing plant (gluten) protein with algae protein. Aquaculture Research published online: 5 MAR 2012 DOI: 10.1111/j.1365-2109.2012.03100.x
Jackson A.E., Ayer S.W., Laycock M.V. (1992). The effect of salinity on growth and amino acid composition in the marine diatom Nitzschia pungens. Canadian Journal of Botany 70, 2198-2201
Lazo J.P., Dinis M.T., Holt G.J., Faula C., Arnold C.R. (2000). Co-feeding microparticulate diets with algae: toward eliminating the need of zooplankton at first feeding in larval red drum (Sciaenops ocellatus). Aquaculture 188, 339-3512
Li P., Mai K, Trushenski J., Wu G. (2009). New developments in fish amino acid nutrition: towards functional and environmentally oriented aquafeeds. Amino Acids 37, 43-53
Lourenço S.O., Barbarino E., De-Paula J.C., da S. Pereira L.O., Lanfer Marquez U.M. (2002). Amino acid composition, protein content and calculation of nitrogen-to-protein conversion factors for 19 tropical seaweeds. Phycological Research 50, 233–241
Miller M.R., Nichols P.D., Carter C.G. (2008). n-3 Oil sources for use in aquaculture – alternatives to the unsustainable harvest of wild fish. Nutrition Research Reviews 21, 85-96
Murata M., Nakazoe J. (2001). Production and use of marine algae in Japan. Japan Agricultural Research Quarterly 35, 281-290
Mustafa M.G., Wakamatsu S., Takeda T. (1995). Effect of algae as a feed additive on growth performance in red sea bream, Pagrus major. Trace Nutrients Research 12, 67–72
Pickova J., Mørkøre T. (2007). Alternate oils in fish feeds. European Journal of Lipid Science and Technology 109, 256–263
Ortiz J., Romero N., Robert P., Araya J., Lopez-Hernández J., Bozzo C., Navarrete E., Osorio A., Rios A. (2006). Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea antarctica. Food Chemistry 99, 98–104
Rocha R.J., Ribeiro L., Costa R., Dinis M.T. (2008). Does the presence of microalgae influence fish larvae prey capture? Aquaculture Research 39, 362 – 369
Rosell K.-G., Srivastava L.M. (1985). Seasonal variations in total nitrogen, carbon and amino acids in Macrocystis integrifolia and Nereocystis luetkeana (Phaeophyta). Journal of Phycology 21, 304–309
Spolaorea P., Joannis-Cassan C., Duran E., Isambert A. (2006). Commercial applications of microalgae. Journal of Bioscience and Bioengineering 101, 87–96
Tacon A.G.J., Metian M. (2008). Global overview on the use of fish meal and fishoil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 285, 146–158
Tartiel M.B., Ibrahim E.M., Zeinhom M.M. (2008). Partial replacement of fish meal with dried microalga (Chlorella spp and Scenedesmus spp) in Nile Tilapia (Oreochromis niloticus) diets. 8th International Symposium on Tilapia in Aquaculture, pp. 801-811
Valente L.M.P., Gouveia A., Rema P., Matos J., Gomes E.F., Pinto I.S. (2006). Evaluation of three seaweeds Gracilaria bursa-pastoris, Ulva rigida and Gracilaria cornea as dietary ingredients in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 252, 85–91
Van Der Meeren T., Mangor-Jensen A., Pickova J. (2007). The effect of green water and light intensity on survival, growth and lipid composition in Atlantic cod (Gadus morhua) during intensive larval rearing. Aquaculture 265, 206-2173
Walker A.B., Fournier H. R., Neefus C.D., Nardi G.C., Berlinsky D.L. (2009). Partial replacement of Fish Meal with Laver Porphyra spp. in diets for Atlantic Cod. North American Journal of Aquaculture 71, 39-45
Walker A.B., Sidor I.F., O'Keefe T., Cremer M., Berlinsky D.L. (2010). Effects of replacement of fish meal protein by microalgae on growth, feed intake, and body composition of Atlantic Cod. North American Journal of Aquaculture 72, 343-353
Wassef E.A., El Masry M.H., Mikhail F.R. (2001). Growth enhancement and muscle structure of striped mullet, Mugil cephalus L., fingerlings by feeding algal meal-based diets. Aquaculture Research 32, 315–322
Wassef E.A., El-Sayed A.F.M., Kandeel K.M., Sakr E.M. (2005). Evaluation of Pterocladia (Rhodophyta) and Ulva (Chlorophyta) Meals as additives to Gilthead Seabream Sparus Aurata diets. Egyptian Journal Of Aquatic Research 31, Special Issue, 321-332
Wong K.H., Peter C.K. (2000). Nutritional evaluation of some subtropical red and green seaweeds: Part I — proximate composition, amino acid profiles and some physico-chemical properties. Food Chemistry 71, 475–482
Yone Y., Furuichi M., Urano K. (1986). Effects of dietary wakame Undaria penatifida and Ascophyllum nodosum supplements on growth, feed efficiency, and proximate compositions of liver and muscle of red sea bream [Chrysophrys major]. Bulletin of the Japanese Society of Scientific Fisheries 52, 1465-1468
Related topics
Authors:
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post24 de mayo de 2021
Good afternoon I am a marine biologist of chili and I work with edible algae, I would like to have a copy of their work related to the use of algae for food and when they come to chili they must warn me to take a tour all over the coast.
10 de febrero de 2014
obously unicellular green algae is easy to digetive to alternate of other chmical nutritions because in stage of larvae it is easly digestive. it is natural feed for fish larvae.


