Morphological and biometric characterisation of rare males and sexual dimorphism in parthenogenetic Artemia (Crustacea: Anostraca)
Published: November 30, 2012
By: Alireza Asem, Behrooz Atashbar, Nasrullah Rastegar-Pouyani, Naser Agh
The genus Artemia Leach, 1819 is a complex taxon with both sexual species and asexual populations. The asexual populations are considered as biological species "Artemiaparthenogenetica Barigozzi, 1974" (BROWNE & BOWEN 1991). Since the reproductive mechanism of parthenogenetic Artemia is via parthenogenesis, males are rarly produced among these individuals. Therefore, they are introduced nowadays as "parthenogenetic Artemia populations" (ABATZOPOULOS et al. 2002), but sexual populations of Artemia have sexual reproduction and their offspring have an equal frequency of males and females.
We studied the morphological and biometric features of males and the sexual dimorphism in parthenogenetic populations of Artemia at Urmia Lake, Iran. The lake basin contains bisexual Artemia species: A. urmiana Günther, 1899 and parthenogenetic Artemia populations within Urmia Lake and other, parthenogenetic population from lagoons around Urmia Lake (ABATZOPOULOS et al. 2006; ASEM et al. 2009). Morphological studies show that two parthenogenetic Artemia from the Urmia Lake basin (costal lagoon and within the lake) can be attributed to two populations (ASEM et al. 2009).
For our study, the cyst samples of the parthenogenetic population of Urmia Lake have been taken from the Artemia and Aquatic Animal Institute, Urmia University, Iran. After hatching, nauplii were cultured at 80 ppt salinity under standard conditions in laboratory (AMAT et al. 2005). 30 mals were collected over five months, with less than 0.4% frequency and were compared with 30 females.
Generally, there are two main shapes for the frontal knob in bisexual species of Artemia: while A. salina (Linnaeus, 1758) is characterised by having a subconical frontal knob, the other species belonging to the genus Artemia have a subspherical frontal knob (MURA & BRECCIAROLI 2004). The morphological structure of frontal knob in the males of the parthenogenetic population from Urmia Lake is recognised by its subspherical shape (Fig. 1a). The furca also appears to vary in its morphology, being two-lobed with many setae or rudimentary with few setae. Individuals of this population have a two lobed furca with many setae (Fig. 1b).
Table 1. Morphometric and meristic characters for males and females of Artemia. The table gives mean (mm) and Standard Deviation (S.D.).
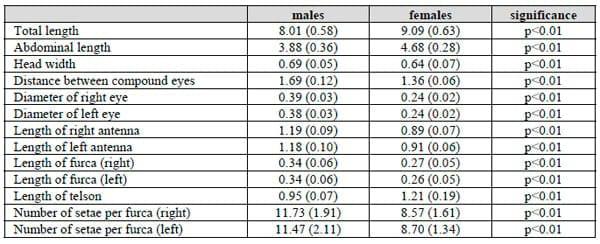
Fig. 1. The structure of frontal knob: subspherical shape (left) and furca: tow-lobed with many setae (right) in a parthenogenetic Artemia population from Urmia Lake.
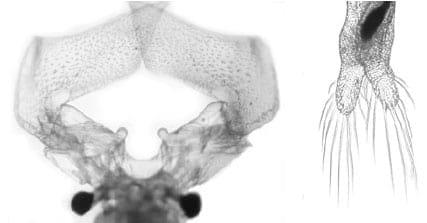
Fig. 2. Scatterplot of Principal Components Analysis in males and females of the parthenogenetic Artemia from Urmia Lake.
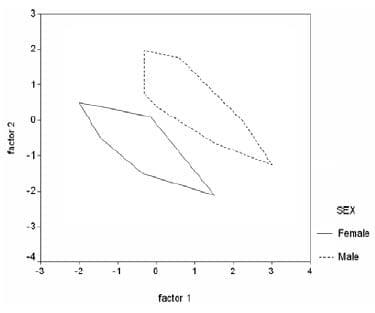
Twelve morphometric characters which are common between males and females were measured (ASEM & RASTEGAR-POUYANI 2007, see also ZHOU et al. 2003, CAMARGO et al. 2003, AMAT et al. 2005). The means of morphometric and meristic characters in males of parthenogenetic Artemia and their significant differences with females are shown in Table, 1 (t-Test, p<0.01). Principal Components Analysis shows that male and female groups are completely separated (Fig. 2). The first and second components show 66.45% and 14.56% of the total variation respectively; in total the two components show 81.02% of the variation. Discriminant Function Analysis confirms 100% of the original groupings.
Bisexual species of Artemia show Reversed Sexual Dimorphism (RSD) in that the female is significantly larger than the male (ASEM & RASTEGAR-POUYANI 2007). In most animals, the male is the larger sex, so dimorphism has been attributed to sexual selection for larger males and the competitive advantages which this confers on them during competition for females (ANDERSSON 1994). A size difference between sexes can be interpreted as a mating advantage because, so far as the Artemia breeding system is concerned, the female carries the male during copulation. For this reason the female needs a larg body for this mating Procedure and for surviving the mating process. However parthenogenetic populations of Artemia have independent reproductive processes, without the males. But this result can prove that at less parthenogenetic population of Artemia from Urmia Lake has retained its ancestral and evolutionary characters because the parthenogenetic Artemia populations are new communities that have been separated from bisexual species of Artemia (BEARDMORE & ABREU-GROBOIS 1983, BAXEVANIS el al. 2006).
Acknowledgements. This study was funded by the Artemia and Aquatic Animals Research Institute of Urmia University, Iran.
References
ABATZOPOULOS, T. J., N. AGH, G. VAN STAPPEN, S. M. RAZAVI ROUHANI & P. SORGELOOS (2006): Artemia sites in Iran – Journal of the Marine Biological Association of the United Kingdom 86: 299-307.
ABATZOPOULOS, T. J., J. A. BEARDMORE, J. S. CLEGG & P. SORGELOOS (2002): Artemia: Basic and Applied Biology. – Dordrecht.
AMAT, F, F. HONTORIA, O. RUIZ, A. J. GREEN, M. I. SÁNCHEZ, J. FIGUEROLA & F. HORTAS (2005): The American brine shrimp as an exotic invasive species in the western Mediterranean. – Biological Invasions 7: 37-47.
ASEM, A, B. ATASHBAR & N. RASTEGAR-POUYANI (2009): Biometrical comparison of two parthenogenetic populations Artemia Leach, 1819 from the Urmia Lake basin, Iran (Anostraca: Artemiidae). – Zoology in the Middle East 47: 117-120.
ASEM, A. & N. RASTEGAR-POUYANI (2007): Sexual Dimorphism in Artemia urmiana Günther, 1899 (Anostraca: Artemiidae) from the Urmia Lake, West Azerbaijan, Iran. – Journal of Animal and Veterinary Advances 6: 1409-1415.
BAXEVANIS, A. D., I. KAPPAS & T. J. ABATZOPOULOS (2006): Molecular phylogenetics and asexuality in the brine shrimp Artemia. – Molecular Phylogenetics and Evolution 40: 724-738.
BEARDMORE, J. A. & F. A. ABREU-GROBOIS (1983): Taxonomy and evolution in the brine shrimp Artemia. p. 153-164. In: G. S. OXFORD & D. ROLLINSON (Eds), Protein Polymorphism: Adaptive and Taxonomic Significance. – London.
BROWNE, R. A. & S. T. BOWEN (1991): Taxonomy and population genetics of Artemia. p. 221-253. In: R. A. BROWNE, P. SORGELOOS &C. A. N. TROTMAN (Eds), Artemia Biology. – Boca Raton (Florida).
CAMARGO, W. N., J. S. ELY & P. SORGELOOS (2003): Morphometric characterization of thalassohaline Artemia franciscana populations from the Colombian Caribbean. – Journal of Biogeography 30: 697-702.
MURA, G. & B. BRECCIAROLI (2004): Use of morphological characters for species separation within the genus Artemia (Crustacea, Branchiopoda). – Hydrobiologia 520: 179-188.
ZHOU, K., M. XU & X. YIN (2003): Morphological characterization of sexual Artemia (Branchiopoda) from China. – Crustaceana 76: 1331-1346.
Related topics
Authors:
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post

