Cadmium in Tilapia
Amelioration the Toxic Effects of Cadmium-Exposure in Nile Tilapia (Oreochromis Niloticus) by using Lemna gibba L
Published: January 25, 2012
By: Hussein A. Kaoud, Manal Moustafa Zaki, Ahmed R. El-Dahshan, Sherein Saeid and Hesham Y. El Zorba (Cairo University)
Abstract
The effect of cadmium (Cd) toxicity and its impact on histopathology, haematological, and biochemical changes in Nile tilapia (Oreochromius niloticus) were studied. Fish (35-45 grams) were exposed to Cd that resulted in significant reduction (p< 0.05) of the erythrocyte count (RBCs), haemoglobin content (Hb) and haematocrit value (Hct). Significant increases in plasma aspartate aminotranseferase (AST) and alanine aminotransferase (ALT) were observed in fish exposed to Cd. The obtained results indicate that Lemna gibba L weed and their extract are effective in removing Cd from water and reducing Cd bioaccumulation in fish. The addition of Lemna gibba L-extract reduced significantly (p<0.05) the Cd level in water and the metal uptake as compared to fish exposed to Cd alone.
The Cd concentration in water was 9.620±0.198 mg L-1 and it decreased significantly (p< 0.05). The Cd accumulation in liver and muscles of fish exposed to Cd alone was higher than that of Lemna gibba L-extract treatment. The addition of Lemna gibba L-extract improves the haematological parameters (RBCs, Hb and Hct) and ameliorates the toxic effect of Cd which indicating the capability of Lemna gibba L-extract to chelate Cd from the media. Subsequently, the Cd toxicity was reduced. This study indicates that Cd poisoning cause structural damage in the fish organs. It is also demonstrated that Lemna gibba L-extract, weed or the weed plus the extract provided protection against the degenerative action of Cd and increased the chance of tissue regeneration.
Keywords: Cadmium; histopathology; Lemna gibba L; amelioration; Oreochromis niloticus
The Cd concentration in water was 9.620±0.198 mg L-1 and it decreased significantly (p< 0.05). The Cd accumulation in liver and muscles of fish exposed to Cd alone was higher than that of Lemna gibba L-extract treatment. The addition of Lemna gibba L-extract improves the haematological parameters (RBCs, Hb and Hct) and ameliorates the toxic effect of Cd which indicating the capability of Lemna gibba L-extract to chelate Cd from the media. Subsequently, the Cd toxicity was reduced. This study indicates that Cd poisoning cause structural damage in the fish organs. It is also demonstrated that Lemna gibba L-extract, weed or the weed plus the extract provided protection against the degenerative action of Cd and increased the chance of tissue regeneration.
Keywords: Cadmium; histopathology; Lemna gibba L; amelioration; Oreochromis niloticus
1. Introduction:
Cadmium (Cd) is a well known heavy metal toxicant with a specific gravity 8.65 times greater than water (Lide, 1992). Cadmium is an extremely toxic metal. It is widely used in mining, metallurgical operations, electroplating industries manufacturing vinyl plastics which used in metallic and plastic pipes. Effluents from such activities are sources of cadmium into aquatic environments. Most aquatic organisms have the capability of concentrating metals by feeding and metabolic processes, which can lead to accumulation of high concentrations of metals in their tissues. Metals interact with legends in proteinsparticularly, enzymes and may inhibit theirbiochemical and physiological activities (Passow et al., 1961).
The levels of contamination by cadmium in fish are of considerable interest because fish consumption is an important source of intake cadmium for the general population. Most of the cadmium content in fish or other seafood is highly absorbable in humans; the efficiency of gastrointestinal absorption of cadmium has been reported to be approximately 3-8% of the ingestedload. Cadmium is particularly accumulated in kidney and to lower extent in muscles (ATSDR, 2003).
Bio-enhancement of Cd transfer along a food chain was studied by Seebaugh & Wallace (2005) and fish are reported to be used as biological indicators to assess water pollution (Rashed, 2001). In aquatic systems, as fish occupy the upper trophic level, there are greater chances of transferring Cd to higher organisms particularly to human. The European Commission (2001) established the maximum levels permitted of cadmium in seafood as follows 0.05 mg/kg in fish, 0.5 mg/kg in crustaceans (except crab), 1.0 mg/kg in molluscs and crab. Moreover, the Joint FAO/ World Health Organization have recommended the provisional tolerable weekly intake (PTWI) as 0.007 mg/kg for cadmium (0.420 mg/g/week for a 60-kg person) (FAO/WHO, 2005).
Metal bioaccumulation can occur via complexation, coordination, chelating, ion exchange and other processes of greater or lesser specificity. It is a strictly aggressive process in which metal ions are sequestered by metal binding site in the interior of the cell. The removal of toxic elements from contaminated water has potential advantages over the conventional treatment process. The reduction of toxic elements like cadmium in aquatic environments is needed by any acceptable method. The most widely used technique for the removal of toxic elements involves the process of neutralization and metal hydroxide precipitation (Hiemesh & Mahadevaswamy, 1994).
The use of aquatic plants in water ecosystems and terrestrial plants in hyonic systems has high potential to clean up the metal contaminated water through phytoextraction and phytostabilization. Phytostabilization utilizes the plant production of compounds, which immobilize contaminants at the entrance of roots. An example of this method is where root exudates cause the precipitation of metals and reducing their bioavailability. Phytodegradation (also known as phytotransformation) is the enzyme-catalysed metabolism of contaminants, typically organics, within plant tissues. The enzymes are usually dehalogenases, oxygenases and reductases (Black, 1995).
Biosorption potential of Prosopis juliflora seed powder (PJSP) for Pb (II) from aqueous solution has been investigated by Jayaram & Prasad (2009) where they found that the maximum Pb (II) adsorbed was found to be 40.322 mg/g and the adsorption process was spontaneous and exothermic in nature. Removal of certain heavy metals from waste water by Lemna gibba L. has been reported by Kwan & Smith (1991); Buckley (1994); Miranda & IIangovan (1996) and Wafaa (2007).
Cadmium (Cd) is a well known heavy metal toxicant with a specific gravity 8.65 times greater than water (Lide, 1992). Cadmium is an extremely toxic metal. It is widely used in mining, metallurgical operations, electroplating industries manufacturing vinyl plastics which used in metallic and plastic pipes. Effluents from such activities are sources of cadmium into aquatic environments. Most aquatic organisms have the capability of concentrating metals by feeding and metabolic processes, which can lead to accumulation of high concentrations of metals in their tissues. Metals interact with legends in proteinsparticularly, enzymes and may inhibit theirbiochemical and physiological activities (Passow et al., 1961).
The levels of contamination by cadmium in fish are of considerable interest because fish consumption is an important source of intake cadmium for the general population. Most of the cadmium content in fish or other seafood is highly absorbable in humans; the efficiency of gastrointestinal absorption of cadmium has been reported to be approximately 3-8% of the ingestedload. Cadmium is particularly accumulated in kidney and to lower extent in muscles (ATSDR, 2003).
Bio-enhancement of Cd transfer along a food chain was studied by Seebaugh & Wallace (2005) and fish are reported to be used as biological indicators to assess water pollution (Rashed, 2001). In aquatic systems, as fish occupy the upper trophic level, there are greater chances of transferring Cd to higher organisms particularly to human. The European Commission (2001) established the maximum levels permitted of cadmium in seafood as follows 0.05 mg/kg in fish, 0.5 mg/kg in crustaceans (except crab), 1.0 mg/kg in molluscs and crab. Moreover, the Joint FAO/ World Health Organization have recommended the provisional tolerable weekly intake (PTWI) as 0.007 mg/kg for cadmium (0.420 mg/g/week for a 60-kg person) (FAO/WHO, 2005).
Metal bioaccumulation can occur via complexation, coordination, chelating, ion exchange and other processes of greater or lesser specificity. It is a strictly aggressive process in which metal ions are sequestered by metal binding site in the interior of the cell. The removal of toxic elements from contaminated water has potential advantages over the conventional treatment process. The reduction of toxic elements like cadmium in aquatic environments is needed by any acceptable method. The most widely used technique for the removal of toxic elements involves the process of neutralization and metal hydroxide precipitation (Hiemesh & Mahadevaswamy, 1994).
The use of aquatic plants in water ecosystems and terrestrial plants in hyonic systems has high potential to clean up the metal contaminated water through phytoextraction and phytostabilization. Phytostabilization utilizes the plant production of compounds, which immobilize contaminants at the entrance of roots. An example of this method is where root exudates cause the precipitation of metals and reducing their bioavailability. Phytodegradation (also known as phytotransformation) is the enzyme-catalysed metabolism of contaminants, typically organics, within plant tissues. The enzymes are usually dehalogenases, oxygenases and reductases (Black, 1995).
Biosorption potential of Prosopis juliflora seed powder (PJSP) for Pb (II) from aqueous solution has been investigated by Jayaram & Prasad (2009) where they found that the maximum Pb (II) adsorbed was found to be 40.322 mg/g and the adsorption process was spontaneous and exothermic in nature. Removal of certain heavy metals from waste water by Lemna gibba L. has been reported by Kwan & Smith (1991); Buckley (1994); Miranda & IIangovan (1996) and Wafaa (2007).
In the present study, short and long-term bioassays were designed to evaluate the influence of Lemna gibba L- plant and/or its extract on the reduction of cadmium in water as well as to investigate the amelioration effect of Lemna gibba L on some blood parameters, enzymes and histopathological alterations induced by Cd exposure on Nile tilapia (Oreochromis niloticus).
2. Materials and Methods:
Fish culture management
Healthy Oreochromis niloticus of 35-45 grams were collected during the late August and early September, 2010 from the ponds of the Central Laboratory for Aquaculture Research at Abbassa, Abo-Hammad, and Sharkia, Egypt (belonging to a single population) .They were collected locally and confined to large plastic aquaria bearing tap water for up to 7 days in the laboratory for acclimation.
Cadmium chloride
Technical grade cadmium chloride (99% purity) was obtained from El-Nasr Chemical Company (Cairo, Egypt) and prepared in aquatic solution to provide the required concentrations of cadmium. Control test without cadmium was performed.
Determination of Lc50
Acute Toxicity Assays
2. Materials and Methods:
Fish culture management
Healthy Oreochromis niloticus of 35-45 grams were collected during the late August and early September, 2010 from the ponds of the Central Laboratory for Aquaculture Research at Abbassa, Abo-Hammad, and Sharkia, Egypt (belonging to a single population) .They were collected locally and confined to large plastic aquaria bearing tap water for up to 7 days in the laboratory for acclimation.
Cadmium chloride
Technical grade cadmium chloride (99% purity) was obtained from El-Nasr Chemical Company (Cairo, Egypt) and prepared in aquatic solution to provide the required concentrations of cadmium. Control test without cadmium was performed.
Determination of Lc50
Acute Toxicity Assays
Laboratory bioassays were conducted to determine the 24 hrs, 48 hrs, 72 hrs and 96 hrs LC50 values for tilapia exposed to CdCl2. The experimental design and calculations for the acute toxicity were based on well-known procedures given by Finney (1978) and Sparks (2000). The tests were carried out in 50 Litres rectangular fibreglass aquaria filled with well-aerated tape water (pH 6.5-7.0). Dissolved oxygen in each tank was maintained at close to saturation by aeration. The temperature in each aquarium was maintained at 25.5-27°C using submerged heaters. The photoperiod was 12 hrs light length/day. The fish were visibly free of any deformities, lesions, or disease and were acclimated in tap water for 1 week prior to the experiment. The nominal concentrations of CdCl2 tested were (control), 1, 2, 5, 10, 20, 40, and 80 mg/ L (Chung, 1983). Gross fish mortality of each concentration was recorded every 1 h for the first 12 hrs andevery 2 hrs thereafter for 96 hrs while the dead fish were removed every 3-8 hrs.
Tilapias were not fed throughout the test. The control and each test concentration were tested in duplicate. LC50 value of cadmium chloride for T. nilotica was determined by the simple graphic method, Probit graphic method and the un-weighted regression method (Finney, 1971).
The tested weed
Tilapias were not fed throughout the test. The control and each test concentration were tested in duplicate. LC50 value of cadmium chloride for T. nilotica was determined by the simple graphic method, Probit graphic method and the un-weighted regression method (Finney, 1971).
The tested weed
The duckweed species used was Lemna gibba L which was taken from Ganabiet-Tersa drain,Giza, Egypt. The duckweed was acclimatized to the laboratory conditions for one week before starting the experiments.
Plant extracts
Dried plant materials were extracted twice with 50% and 100% methanol as well as 50% and 100% acetone in v/v proportions (200 ml/5g plant) for 2 hrs with constant stirring. The collected filtered extracts were dried in a rotary evaporator (Büchi:Rotavapor-R114 and water bath B-481) at 40oC under reduced pressure (Ghobrial et al., 2009).
Cadmium reduction
Tilapias were distributed randomly in 120 Litres rectangular fibreglass aquaria filled with wellaerated tape water (pH 6.5-7.0) at a rate of 15 fish /aquarium. Dissolved oxygen in each tank was maintained at close to saturation by aeration. The temperature in each aquarium was maintained at 27±1°C by means of thermostats. The photoperiod was 12 hrs light-length/days. These aquaria were divided into five groups with three replicates each per group. The first group was free from Cd and Lemna gibba L and maintained as a control. The second groups were exposed to 10 mg of CdCl2 only (Equivalent to 1/4 96 h LC50). The third, fourth and fifth group were exposed to 10 mg CdCl2 L-1 and 0.1, 1 and 0.1 plus 1 g L- 1 extract, plant and extract plus plant of Lemna gibba L, respectively (Table I).
Tilapias were distributed randomly in 120 Litres rectangular fibreglass aquaria filled with wellaerated tape water (pH 6.5-7.0) at a rate of 15 fish /aquarium. Dissolved oxygen in each tank was maintained at close to saturation by aeration. The temperature in each aquarium was maintained at 27±1°C by means of thermostats. The photoperiod was 12 hrs light-length/days. These aquaria were divided into five groups with three replicates each per group. The first group was free from Cd and Lemna gibba L and maintained as a control. The second groups were exposed to 10 mg of CdCl2 only (Equivalent to 1/4 96 h LC50). The third, fourth and fifth group were exposed to 10 mg CdCl2 L-1 and 0.1, 1 and 0.1 plus 1 g L- 1 extract, plant and extract plus plant of Lemna gibba L, respectively (Table I).
Fish were fed frequently on a diet containing 30% crude protein at a rate of 3% of live body weight twice daily for 7 and 25 days. Siphoning three quarters aquariums was done every day for waste removal and replacing it by an equal volume of water containing the same concentration of Cd and Lemna gibba L. Dead fish were removed and recorded daily.
Hematological, enzymatic and histopathological investigations
After 7 and 25 days of the experiment, blood samples were taken from three Fish from each aquarium. Fish were not fed for 24 hrs before sampling and were anaesthetized with buffered MS222 (50 mg L-1) and blood samples were taken from the caudal vein of an anaesthetized fish by sterile syringe containing EDTA solution as anticoagulant. These blood samples were used for determining erythrocyte count (Dacie & Lewis, 1984) and hemoglobin content (Van Kampen & Zijlstra, 1961). Haematocrit value (Hct) was calculated according to the formulae mentioned by Britton (1963).
Plasma was obtained by centrifugation of blood at 3000 rpm for 15 min and non haemolysed plasma was stored in a deep freezer for further biochemical analyses. After decapitation of fish, samples of gills, liver, spleen, kidney, stomach, intestine and brain were taken and frozen for histopathological investigations. Plasma activities of aspartate amninotransferase (AST) and alanine aminotransferase (ALT) were determined calorimetrically according to Reitman & Frankel (1957)
Plasma was obtained by centrifugation of blood at 3000 rpm for 15 min and non haemolysed plasma was stored in a deep freezer for further biochemical analyses. After decapitation of fish, samples of gills, liver, spleen, kidney, stomach, intestine and brain were taken and frozen for histopathological investigations. Plasma activities of aspartate amninotransferase (AST) and alanine aminotransferase (ALT) were determined calorimetrically according to Reitman & Frankel (1957)
Cd residue
Cadmium residues were measured in water, liver and muscles according to method of Eaton & Stinson (1983). The water samples were preserved by the addition of one mL of concentrated nitric acid per litre until the time of analysis. The water samples were filtered through 0.45μl membrane filter. The required volume (100 mL) of the filtrate was collected to measure cadmium levels in water samples by using Air/Acetylene Flame Atomic Absorption Spectrophotometer (UNICAM 696 AA Spectrometer).
The analysis of tissue sample was represented by one gram of tissues dissected from the liver and muscles, then placed in a clean screwcapped tube and digested according to the method described by Finerty et al. (1990). The obtained solutions were then analyzed by using Air/ Acetylene Flame Atomic Absorption Spectrophotometer (UNICAM 696 AA Spectrometer).
Statistical analysis
The obtained data were subjected to analysis of variance according to Snedecor & Cochran (1982). Differences between means were done at the 5% probability level, using Duncan´s new multiple range test (Duncan, 1955).
3. Results:
The LC50 values from the three methods (the simple graphic method, Probit graphic method and the un-weighted regression method) are 40.47 mg/L, 40.99 mg/L and 40.13 mg/L ,respectively. Hence the calculated average LC50 is 40.533 mg/L. The equation for the dose mortality regression line was found to be Y = 2.65X+3.368.
Hematological parameters
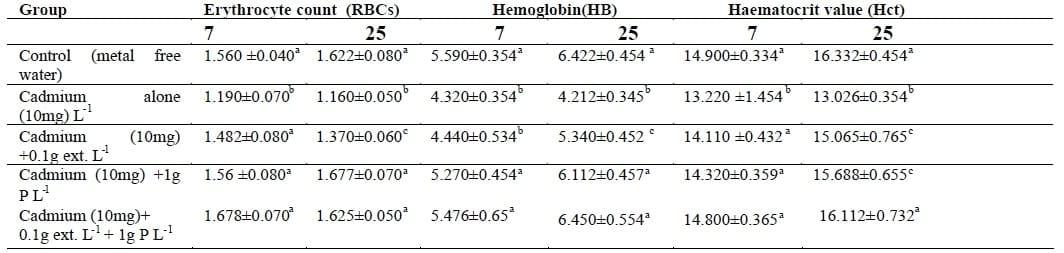
Table II shows that the RBCs , HB and HCT were reduced in fish exposed to Cd at both periods and they were lower than that of the control (p<0.05). The RBCs count also decreased significantly in fish exposed to Cd at 7 and 25 days. On the other hand, these parameters were return to the normal values and increased significantly in fish exposed to Cd with Lemna gibba L- weed and/or its extract for 7 and 25 days. Blood parameters were improved in fish exposed to Cd with different levels of Lemna gibba L.
Biochemical parameters
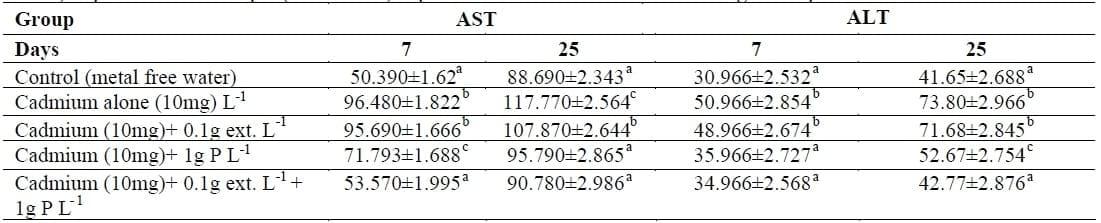
Table III showed that AST activity increased significantly in plasma of fish exposed to Cd alone The addition of Lemna gibba L-extract decreased significantly the AST activity to be lower than that of Cd alone (p<0.05). The AST activity in fish exposed to Cd with Lemna gibba L plus their extract became nearly similar to that of control at 7 days and 25 days. The plasma ALT activity increased significantly in fish exposed to Cd alone at 7 and 25 days. The addition of Lemna gibba L enhanced ALT activity to be nearly as in the control especially the groups exposed to Cd with Lemna gibba L- weed plus their extract at both periods.
Histopathological alterations
The histopathology of different Tilapia tissues revealed that there are several histopathological changes in different Tilapia organs as shown in our figures.
Gills (Figure 1(A)) showed necrosis and atrophy of the gill lamellae. Severe edema, hyperplasia, fusion and focal desquamation of the epithelial lining of the secondary lamellae also were detected. The gill arch showed numerous mononuclear leucocytic infiltration, edema, congestion and the apex of gill filaments showed congestion, hyper activation of the mucous and chloride cells.
Liver of tilapia treated with cadmium showed degeneration of the hepatocytes with nuclear pyknosis in the majority of the cells as well as the accumulation of the metal binding proteins in their nuclei. Intravascular hemolysis is seen in blood vessels and sinusoids with necrosed hepatocytes (Figures 1 (B, C, D, E)).
Spleen The microscopic examination of Tilapia´s spleen revealed lymphoid depletion (LD) associated with congestion and necrosis of splenic sinusoids and activation of Melanomacrophage center (MMC). Large sub-capsular areas of necrosis were also observed (Figure 1(F), 2(A)). In the control fish, liver contains few melano macrophage centers (MMC) and free macrophages.
Spleen The microscopic examination of Tilapia´s spleen revealed lymphoid depletion (LD) associated with congestion and necrosis of splenic sinusoids and activation of Melanomacrophage center (MMC). Large sub-capsular areas of necrosis were also observed (Figure 1(F), 2(A)). In the control fish, liver contains few melano macrophage centers (MMC) and free macrophages.
Stomach The sub-mucosal tissues were fully vacuolated with degeneration of the serosal layer, degeneration of columnar epithelium, goblet cell and basement membrane as well as the secretory cells were damaged and fully distorted (Figure 2(B)).
Intestine Intestine of tilapia treated with cadmium showing necrosed mucosa, sub-mucosal hemorrhage, muscle fibers were loosely arranged with the degeneration of sub-mucosal tissue and each villus facing the lumen showed cell degeneration and the cells did not show distinct nuclei and cytoplasmic boundaries. There was a distortion of basement membrane of the villi and blood vessel, and lymphocytes were fully distorted and there was a degeneration of columnar epithelium of the intestine (Figure 2 (C, D, E, F)).
Muscular tissues degeneration in muscle bundles with aggregations of inflammatory cells (leucocytic infiltration) between them with focal areas of necrosis (Figure 2 (F)). Atrophy and edema of muscle bundles as well as splitting of muscle fibers and hyalinized muscles tissue were seen (Figure 3 (A, B)).
Brain edema was the main characteristic histopathological change in the brain tissues. The lesion also characterized by vacuolation of brain tissue in addition to encephalitis that characterized microscopically by congestion of cerebral blood capillaries associated with neuronal degeneration, severe necrosis and demylination of brain tissue with extravasations of free RBCs as well as aggregation of EGCs in brain tissue (Figure 3 (C, D, E, F)).
Kidney showed hyic swelling of the renal tubules, sometimes with pyknotic nuclei and many necrotic areas as well as swollen proximal epithelial cells with necrotic nuclei (Figure 4 (A)).
Abnormal blood cells Tilapia treated with cadmium showing abnormal blood cells (Aminocytosis) (Figure 4 (B, C)). Gills of tilapia treated with cadmium showing atrophy and necrosis of gill lamellae (arrows) (H & E X 400).
Cd Bioaccumulation
The highest bioaccumulation of cadmium was observed in the organs mainly implicated in metal intoxication and so it was higher in the liver followed by muscles.
Addition of Lemna gibba L-extract to the Cd polluted media reduced significantly (p<0.05) the Cd level in aquarium´s water as compared to that of Cd alone, Table I. The Cd concentration in water exposed Cd alone was 9.620±0.198 mg L-1 and declined significantly (p<0.05) to 7.8 ±0.276, 4.58 ±0.208 and 0.89 ±0.105 mg L-1 with 0.1, 1 and 0.1 plus 1 g L-1 extract, weed and extract plus weed of Lemna gibba L, respectively. The highest amount of Cd residue was found in the liver after 7 days of exposure. Table I also, showed that the uptake of Cd in the liver of fish exposed to Cd alone was 2.350±0.18 and 5.88±0.540 mg g-1 dry weight for 7 and 25 days, respectively. It declined significantly to 1.392±0.095, 4.500±0.320 and 0.990±0.015, 3.792 ±0.320 and 0.510 ±0.021, 1.850 ±0.150 mg g-1 dry weight in fish group exposed to Cd with 0.1, 1 and 0.1 plus 1 g L-1 extract, weed and extract plus weed of Lemna gibba L at 7 and 25 days, respectively. Similar trends were observed in fish muscles.
4. Discussion:
The present study reveals that the fish exposed to Cd alone showed significant reduction in RBCs, Hb and HCT than those exposed to Cd with different level of Lemna gibba L-extract and plant. The reduction of these parameters in O niloticus at sub-lethal levels of cadmium might be due to the destruction of mature RBCs and the inhibition of erythrocyte production due to reduction of haemsynthesis that affected by pollutants (Wintrobe, 1978). Also, the decrease in RBCs count may be attributed to haematopathology that results in sever anemia in most vertebrates including fish species exposed to different environmental pollutants (Khangarot & Tripathi, 1991). The decrease in the RBCs may be attributed to reduction of growth and other food utilization parameters which results in sever anemia (James and Sampath, 1999). Also Gill & Epple (1993) found a significant reduction in the RBCs, Hb and HCT in American eel (Anguilla rostrata) after exposure to 150 ug Cd L-1. Karuppasamy et al. (2005) found a significant decrease in total erythrocyte count, haemoglobin content, haematocrit value and mean corpuscular haemoglobin concentration in air breathing fish, Channa punctatus after exposure to sub-lethal dose of Cd (29 mg Cd L-1).
The present study reveals that the fish exposed to Cd alone showed significant reduction in RBCs, Hb and HCT than those exposed to Cd with different level of Lemna gibba L-extract and plant. The reduction of these parameters in O niloticus at sub-lethal levels of cadmium might be due to the destruction of mature RBCs and the inhibition of erythrocyte production due to reduction of haemsynthesis that affected by pollutants (Wintrobe, 1978). Also, the decrease in RBCs count may be attributed to haematopathology that results in sever anemia in most vertebrates including fish species exposed to different environmental pollutants (Khangarot & Tripathi, 1991). The decrease in the RBCs may be attributed to reduction of growth and other food utilization parameters which results in sever anemia (James and Sampath, 1999). Also Gill & Epple (1993) found a significant reduction in the RBCs, Hb and HCT in American eel (Anguilla rostrata) after exposure to 150 ug Cd L-1. Karuppasamy et al. (2005) found a significant decrease in total erythrocyte count, haemoglobin content, haematocrit value and mean corpuscular haemoglobin concentration in air breathing fish, Channa punctatus after exposure to sub-lethal dose of Cd (29 mg Cd L-1).
The decrease in RBCs, Hb and HCT values may be due to the exaggerated disturbances that occurred in both metabolic and hemopoietic activities of fish exposed to sub-lethal concentration of pollutants (Moussa, 1999).
The activity of AST and ALT enzymes in blood may also be used as a stress indicator. The significant changes in the activities of these enzymes in blood plasma indicate tissue impairment caused by stress (James et al., 1991; Svoboda, 2001). In the present study, there were significant changes in AST and ALT activities in plasma of fish exposed to cadmium compared to the control group. The increase in concentration of AST and ALT in blood plasma indicates impairment of parenchymatous organs mainly liver. In addition, the increase of plasma AST and ALT may be attributed to the hepatocellular damage or cellular degradation in liver, spleen or muscles (Yamawaki et al., 1986).
These results are in agreement with those reported by Shalaby (1997) who found that sub-lethal concentration of Cd caused significant increases in AST and ALT of Common Carp after 7 and 25 days.
Histopathological biomarkers have been largely used in fish to identify and evaluate the toxic effects of pollutants exposure (Rabitto et al., 2005; Oliveira Ribeiro et al., 2006). The presence of necrosis is in fact one of the most visible damages in tissues affected by a pollutant (Rabitto et al., 2005). According to Manahan (1991) the occurrence of necrosis is also a consequence of enzymatic inhibition, damages in the cellular membrane integrity, and disturbances in the synthesis of proteins and carbohydrate metabolism.
Mallat (1985) reported that the pathological changes of fish gills are induced by elevated heavy metals and low oxygen content in water. The hyperplasia induced by any pollutant may be due to the simple response to cellular necrosis as previously mentioned by Marie et al. (1998). Moreover, Shaker et al. (2000) reported that the epithelial hyperplasia is known as a protective and defense mechanism of fish gills. In addition to, the congestion in branchial blood vessels may be due to the counter irritation and stimulation of vasodilators (Marie et al., 1998).
Structural damage due to Cd toxicity in fish gills has been reported by Voyer (1975); Karlson- Norrgren et al. (1985) and Kothari & Saxena (1988). Narayan & Singh (1991) observed extensive degeneration of cytoplasm with pyknosis of nuclei and loss of glycogen in liver tissue of Heteropneustes fossils while subjecting them to acute thiodan toxicity. Recent studies have demonstrated the links between exposure to pollutants and the development of hepatic lesions. Stentiford et al. (2003) and Stehr et al. (2004) observed that toxicopathic liver lesions in fish species are effective biological markers of chemical exposures.
Melano macrophages are pigmented cells that can appear isolated or arranged in clusters forming the MMCs in the spleen. These cells are positive indicator for the presence of neutral carbohydrates (P.A.S. stain) and melanin (Masson- Fontana stain) (Rabitto et al., 2005).
The pathological findings in the intestine included atrophy in the muscularis, degenerative and necrotic changes in the intestinal mucosa and submucosa with necrotized cells aggregated in the intestinal lumen, edema and atrophy in the submucosa. These findings are similar in Chana punctatus exposed to Cadmium (Stromberg et al., 1983) and lead (Sastri & Gupta, 1978).
The present results indicate that Lemna gibba L weed and extract are effective in removing Cd from water and reducing Cd bioaccumulation inTilapia fish. The addition of Lemna gibba L-extract reduced significantly (p<0.05) the Cd level in water and the metal uptake as compared to fish exposed to Cd alone. The Cd concentration in water was 9.620±0.198 mg L-1 and it decreased significantly (p< 0.05). The Cd accumulation in liver and muscles of fish exposed to Cd alone was higher than that of Lemna gibba L-extract treatment group. These results suggest that Lemna gibba L weed and/or extract could chelate Cd ions producing a stable complex, thus reducing the chance for metal uptake by tissues. These results are in agreement with Santschi (1988) who reported that any agent that can remove Cd from water helps to reduce the bioaccumulation of this metal in fish.
The addition of Lemna gibba L-extract improves the haematological parameters (RBCs, Hb and Hct) and ameliorates the toxic effect of Cd in Tilapia fish which indicating the capability of Lemna gibba L-extract to chelate Cd from the media. Subsequently, the Cd toxicity was reduced. These results are in agreement with those recorded by Jayaram & Prasad (2009) who observed the biosorption potential of Prosopis juliflora seed powder (PJSP) for Pb (II) from aqueous solution at pH 6.0. Findings in fish also indicated that degenerative changes were less when Lemna gibba Lextract or the weed and their extract were added to the rearing water.
The present study shows that addition of Lemna gibba L- weed and/or its extract to Cd contaminated media reduced significantly the Cd level in the water and helped to eliminate metal from the fish body and in turn improved the biochemical parameters as compared to fish exposed to Cd alone.
Finally, we could conclude that Cd poisoning cause structural damage in Tilapia organs. It is also demonstrated that Lemna gibba L-extract, weed or the weed plus the extract provided protection against the degenerative action of Cd and increased the chance of tissue regeneration.
Table I: Changes in cadmium residue in water (mg Cd L-1), liver and muscles (mg Cd g-1 dry weigh) of Nile tilapia (O. niloticus) exposed to Cd with and without Lemna gibba L plant.
The same letter in the same column is not significantly different at P<0.05.
The first group was free from Cd and Lemna gibba L and maintained as a control.
The second groups were exposed to 10 mg of CdCl2 only (Equivalent to 1/4 96 h LC50).
The third was exposed to 10 mg Cd L-1 and 0.1 extract.
The fourth group was exposed to 10 mg Cd L- 1 and 1 g L- 1Lemna gibba L plant.
The fifth group was exposed to 10 mg Cd L-1+ 0.1g extract L1 +1 g L-1 Lemna gibba L plant.
Table II: Changes in erythrocyte (count x106/mm3), hemoglobin content (g 100 mL-1) and haematocrit value (%) in the blood of Nile tilapia (O. niloticus) exposed to Cd with and without Lemna gibba L plant.
The same letter in the same column is not significantly different at P<0.05.
The first group was free from Cd and Lemna gibba L and maintained as a control.
The second groups were exposed to 10 mg of CdCl2 only (Equivalent to 1/4 96 h LC50).
The third was exposed to 10 mg Cd L-1 and 0.1 extract.
The fourth group was exposed to 10 mg Cd L-1 and 1 g L-1Lemna gibba L plant.
The fifth group was exposed to 10 mg Cd L-1+ 0.1g extract L1 +1 g L-1Lemna gibba L plant.
Table III: Changes in aspartate aminotransferase activity (AST) and alanine aminotransferase (ALT) activity (IU L-1) in plasma of Nile tilapia (O. niloticus) exposed to Cd with and without Lemna gibba L plant.
The same letter in the same column is not significantly different at P<0.05.
The first group was free from Cd and Lemna gibba L and maintained as a control.
The second groups were exposed to 10 mg of CdCl2 only (Equivalent to 1/4 96 h LC50).
The third was exposed to 10 mg Cd L- 1 and 0.1 extract.
The fourth group was exposed to 10 mg Cd L-1 and 1 g L-1 Lemna gibba L plant.
The fifth group was exposed to 10 mg Cd L-1+ 0.1g extract L1 +1 g L-1 Lemna gibba L plant.
Figure 1.
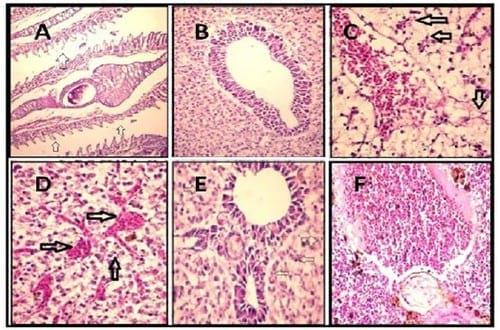
A. Gills of tilapia treated with cadmium showing atrophy and necrosis of gill lamellae (arrows) (H & E X 400).
B. Liver of tilapia treated with cadmium and Plant-ext showing apparently healthy liver tissue (H & E X 200).
C. Liver of tilapia treated with cadmium showing congestion (C) with severely degenerated and necrosed hepatocytes (arrows) (H & E X 200).
D. Liver of tilapia treated with cadmium showing congested sinusoids with necrosed hepatocytes (arrows) (H & E X 200).
E. Liver of tilapia treated with cadmium and Plant-ext showing normal portal tract with slightly degenerated hepatocytes (arrows) (H & E X 400).
F. Spleen of tilapia treated with cadmium and Plant-ext showing apparently normal spleen (H & E X 200).
B. Liver of tilapia treated with cadmium and Plant-ext showing apparently healthy liver tissue (H & E X 200).
C. Liver of tilapia treated with cadmium showing congestion (C) with severely degenerated and necrosed hepatocytes (arrows) (H & E X 200).
D. Liver of tilapia treated with cadmium showing congested sinusoids with necrosed hepatocytes (arrows) (H & E X 200).
E. Liver of tilapia treated with cadmium and Plant-ext showing normal portal tract with slightly degenerated hepatocytes (arrows) (H & E X 400).
F. Spleen of tilapia treated with cadmium and Plant-ext showing apparently normal spleen (H & E X 200).
Figure 2.
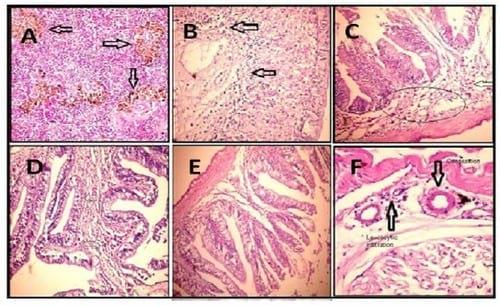
A. Spleen of tilapia treated with cadmium showing hyperplasia in the melanomacrophage cells (arrows) (H & E X 200).
B. Stomach of tilapia treated with cadmium showing leucocytic infiltration in the sub mucosa (arrow) together with congestion (C) (H & E X 400).
C. Intestine of tilapia treated with cadmium showing necrosed mucosa (n), sub mucosal haemorrhage (arrow) (H & E X 200).
D. Intestine of tilapia treated with cadmium showing higher power of the previous lesion (H & E X 400).
E. Intestine of tilapia treated with cadmium and Plant-ext showing apparently intestinal tissue (H & E X 200).
F. Muscles of tilapia treated with showing congestion (C) and leucocytic infiltration (arrow) (H & E X 200).
Figure 3.
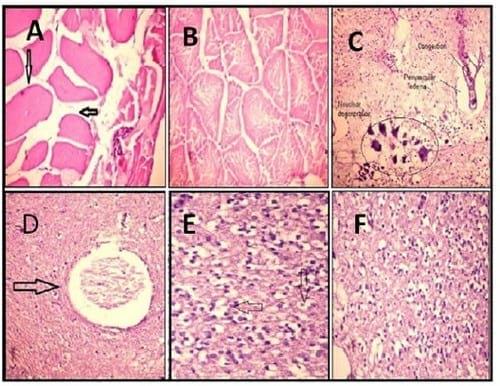
A. Muscles of tilapia treated with cadmium showing hyalinised muscles tissue (arrow) (H & E X 200).
B. Muscles of tilapia treated with cadmium and Plant-ext showing apparently healthy muscular tissue (H & E X 200).
C. Brain of tilapia treated with cadmium showing congestion (C), perivascular edema (e) and neural degeneration (n)(arrow) (H & E X 200).
D.Brain of tilapia treated with cadmium showing osteomalacia (arrow) (H & E X 200).
E. Brain of tilapia treated with cadmium showing intracellular brain edema (arrow) (H & E X 200).
F. Brain of tilapia treated with cadmium and Plant-ext showing apparently healthy tissue (H & E X 200).
Figure 4.
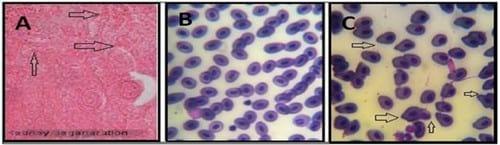
A. Kidneys showed hyic swelling of tubules with pyknotic nuclei and many necrotic areas as well as swollen proximal epithelial cells with necrotic nuclei.
B. Tilapia treated with cadmium and Plant-ext showing apparently healthy blood cells (X 1000).Tilapia treated with cadmium showing abnormal blood cells (Amioscytosis) (X 1000).
B. Tilapia treated with cadmium and Plant-ext showing apparently healthy blood cells (X 1000).Tilapia treated with cadmium showing abnormal blood cells (Amioscytosis) (X 1000).
5. References:
1. ATSDR. (2003). Toxicological Profile for Selenium. Atlanta, GA:Agency for Toxic Substances and Disease Registry.
2. Black, H. (1995). Absorbing possibilities:phytoremediation. Environmental health perspectives 103, 1106-1108.
3. Britton, C. L. (1963). Disorders of the Blood. 9th ed. Achur-Chill. Ltd., London.
4. Buckley, J.A. (1994). The bioavailability of copper in wastewater to Lemna minor with biological and electrochemical measures of complexation. Water research 28, 2457-2467.
5. Chung, K.S. (1983). Lethal Effects of cadmium in Tropical Fish. Bulletin of the Japanese Society of Fisheries Oceanography 49, 1565-68.
6. Dacie, J.V. & Lewis, S.M. (1984). Practical Hematology. 6th edition. Churchill 7. Livingstone. Edinburgh. London. Melbourne and New York. 22 pp.
8. Duncan, D.B. (1955). Multiple ranges and multiple F test. Biometrics. 11, 1-42.
9. Eaton, D.L. & Stinson, M.D., (1983). Concentration of lead, cadmium, mercury and copper in the cray fish (Pacifasticus leniusculus) obtained from a lake receiving urban runoff. Archive of Environmental Contamination and Toxicolology 12, 693- 700.
10. European Commission. (2001). Commission Regulation (EC) No, 466/2001 of 8 March 2001, setting maximum levels for certain contaminants in foodstuffs.
11.FAO / WHO (Food and Agriculture Organization/World Health Organization). 2005. Summary and conclusions of the sixty-four meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). .
12. Finerty, M.W. Madden, J.D., Feagly, S.E. &Grodner, R.M. (1990). Effect of environs and seasonality on metal residues in tissues of wild and pond-raised Crayfish in southern Louisiana. Archive of Environmental Contamination and Toxicolology 19, 49-55.
13. Finney, D.J. (1971). Probit Analysis. Cambridge University Press.
14. Finney, D.J. (1978). Statistical method in biological assay. London. Griffin.
15. Ghobrial, I., Roccaro, A., Chauhan, D., Aderson, K. & Palladino, M. (2009). Methods of using [3.2.0] heterocyclic compounds and analogs thereof in treating waldenstrom´s macroglobulinemia. Nereus Pharmaceuticals, INC. San Diego, CA, US.
16. Gill, T.S. & Epple, A. (1993). Stress-related changes in the hematological profile of 17. the American eel (Anguilla rostrata).Ecotoxicology and Environmental Safety 25, 227-35.
18. Hiemesh, S. & Mahadevaswamy, M., (1994).Sorption potential of biosorban: For the removal of copper. Indian Journal of Environmental Health 36, 165- 169.
19. James, P., Thorpe, I.J., Kokkinos, N., Morkot, R.& Frankish, J. (1991). Centuries of Darkness:context, methodology and implications.Cambridge Journal Archive 1, 228-35.
20. James, R. & Sampath, K. (1999). Effect of the ion- exchanging agent, Zeolite, on reduction of cadmium toxicity: an experimental study on growth and elemental uptake in Heteropneustes fossilis (Bloch). Journal of Aquaculture in the Tropics 14, 65- 74.
21. Jayaram, K. & Prasad, M.N.V. (2009). Removal of Pb(II) from aqueous solution by seed powder of Prosopis juliflora DC. Journal of Hazardous Materials 169 (1-3), 991-7.
22. Karlson-Norrgren, L., Runn, P., Haux, C. &Forlin, L. (1985). Cadmium-induced changes in gill morphology of zebra fish Brachydanio rerio (Hamilton-Buchaman) and rainbow trout Salmogiardneri, Richardson. Journal of Fish Bioliology 27, 81.
23. Karuppasamy, R., Subathra, S. & Puvaneswari, S. (2005). Haematological responses to exposure to sublethal concentration of cadmium in air breathing fish, Channa punctatus (Bloch). Journal of Environmental Biology 26, 123-8.
24. Khangarot, B.S. & Tripathi, D.M. (1991).Changes in humoral and cell mediated immune responses and in skin and respiratory surfaces of catfish, Saccobranchus fossilis following copper exposure.Ecotoxicology and Environmental Safety 22, 291.
25. Kothari, S. & Saxena, G. (1988). Histological and histochemical studies in the gills of Puntius sophore (Ham.) exposed to cadmium chloride. Journal of Hydrobiologica 27, 81
26. Kwan, K.H.M. and Smith, S., (1991). Some aspects of the kinetics of cadmium and thallium uptake by fronds of Lemna minor L. New Phytol. 117, 91-102.
27. Lide, D. (1992). CRC Handbook of Chemistry and Physics, 73rd Edition 1992. Boca Raton, FL: CRC Press.
28. Mallat, J. (1985). Fish gill structural changes induced by toxicants and other irritants: a statistical review. Canadian Journal of Fisheries and Aquatic Science 42, 630-648.
29. Manahan, S. E. (1991). Water Pollution Environment Chemistry, first ed. Lewis Publishers, London
30. Marie, M.A.S., Haggag, A.M. & EI-Badawy, A.A. (1998). Physiological and biochemical responses of the common carp; Cyprinus carpio to an organophosphorus insecticide Profenofos.Egyptian Journal of Zoology 13, 279-302.
31. Mary, G.G., Omar, S. & Tarek, A. (2007). Potential for finding new bioactive agents from selected aquatic plant extracts against enteric bacteria. Egyptian Journal of Natural Toxins 4, 12-25.
32. Miranda, M. & Ilangovan, K. (1996). Uptake of lead by Lemna gibba L.: Influence on specific growth rate and basic biochemical changes. Bulletin of Environmental Contamination and Toxicology 56, 1000-1007.
33. Moussa, M.A. (1999). Biological and physiological studies on the effect of the gramoxon and stomp herbicides on Nile tilapia (Oreochromis niloticus). Ph.D. Thesis, Department of Zoology, Faculty of Science, Cairo University, 200p.
34. Narayan, A.S. & Singh, B.B. (1991). Histopathological lesions in Heteropneustes fossilis subjected to acute thiodan toxicity. Hydrobiologica 19, 235-243.
35. Oliveira, R., Filipak, C.A. & Neto, F.l. (2006). Haematological findings in neotropical fish Hoplias malabaricus exposed to subchronic and dietry doses of methylmercury, inorganic lead, tributyltin chloride. Environmental Research 101, 74-80.
36. Passow, H., Rothstein, A. & Clarkson, T.W.(1961). The general pharmacology of the heavy metals. Pharmacological Reviews 13,185-224.
37. Rabitto, I.S., Alves Costa, J.R.M., Silva de Assis, H.C., Pelletier, E., Akaishi, F.M., Anjos, A., Randi, M.A.F. & Oliveira, R. (2005). Effects of dietary Pb(II) and tributylin an neotroptical fish Hoplias malabarius: Histopathological and biochemical findings. Ecotoxicology and Environmental Safety 60, 147-156.
38. Rashed, M.N. (2001). Cadmium and lead levels in fish (Tilapia niloticus ) tissues as 39. biological indicator for lake water pollution. Environmental Monitoring and Assessment. 68, 75-89.
40. Reitman, S. & Frankel, S. (1957). Colorimetric determination of glutamic oxaloacetic and glutamic pyruvic transaminases. American Journal of Clinical Pathology 28, 53-56.
41. Santschi, P.H. (1988). Factors controlling the biogeochemical cycle of trace elements in fresh and coastal waters as revealed by artificial radioisotopes. Limnology and oceanography 33,848- 886.
42. Sastri, K.V. & Gupta, P.K. (1978). Chronic mercuric chloride intoxication in digestive system of Channa punctatus. Journal of Toxicology and Environmental Health 4, 777.
43. Shaker, I.M., Ez-EI-Rigal, A. & El Ghobashy, H.(2000). Effects of EDTA on reducing of copper toxicity in water and Oreochromis niloticus. Egyptian Journal of Applied Science 15, 119-131.
44. Shalaby, A.M. (1997). Biochemical and physiological studies on metal contamination in the common carp (Cyprinus carpio L). Ph. D. Thesis, Zagazig University, Faculty of Science, Benha branch, 268p.
45. Seebaugh, D.R. & Wallace, W.G. (2005). Absorption of dietry cadmium by grass shrimp Palaemonetes spp. Collected along an environmental impact ingredients. In: Waldman, JR and Nieder WC (eds). Final report of the Tibor T. Polgar Fellowship Program, 2004, section VIII. Hudson River Foundation, New York P1. 46. Sparks, T. (2000). Statistics in Ecotoxicology. John Wiley and Sons, Ltd., West Sussex, England, 320 p.
47. Stehr, C.M., Myers, M.S., Johnson, L.L., Spencer,S. & Stein, J. E. (2004). Toxicopathic liver lesions in English sole and chemical contaminant exposure in Vancouver harbour, Canadian Marine Environmental Research 57, 55-74.
48. Stentiford, G.D., Evans, M.G., Bateman, K. & Feist, S.W. (2003). Co-infection by a yeast-like organism in Hematodinium-infected European edible crabs Cancer pagurus and velvet swimming crabs Necora puber from the English Channel. Diseases of Aquatic Organisms 54,195-202.
48. Stentiford, G.D., Evans, M.G., Bateman, K. & Feist, S.W. (2003). Co-infection by a yeast-like organism in Hematodinium-infected European edible crabs Cancer pagurus and velvet swimming crabs Necora puber from the English Channel. Diseases of Aquatic Organisms 54,195-202.
49. Svoboda, M. (2001). Stress in fishes (a review). Bulletin VÚRH Vodňany 4, 169- 191. 50. Stromberg, P.C., Ferrante, J.G. & Carter, S. (1983). Pathology of lethal and sublethal exposure of fathead minnows, Pimephales promelas, to cadmium: a model for aquatic toxicity assessment. Journal of Toxicology and Environmental Health 11, 247-259.
51. Snedecor, O.W. & Cochran, W.O. (1982). Statistical Methods. 7th Ed,, Iowa University Press, Ames, Iowa.
52. Van Kampen, E.J. & Zijlstra, W.G. (1961). Standardization of hemoglobin- ometry. II. The hemiglobincyanide method. Clinica Chimica Acta 6, 583.
53. Voyer, R.A. (1975). Effect of dissolved oxygen concentration on the acute toxicity of cadmium to mummichog Fundulus heteroclitus (L.) at various salinities. Transactions of the American Fisheries Society 104, 129.
54. Wafaa, A.E., Ismail, G., Farid, A.E., Tarek, T., & Hammad, D. (2007). Assessment of the Efficiency of Duckweed (Lemna gibba) in Wastewater Treatment. International Journal of Agriculure and Biology 9, 681-687.
55. Wintrobe, M.M. (1978). In: Clinical hematology Henry Kimpton, London, pp. 448.
56. Yamawaki, K., Hashimoto, W., Fujii, K.,Koyama, J., Ikeda,Y. & Ozaki, H. (1986). Hemochemical changes in carp (Cyprinus carpio) exposed to low cadmium concentrations. Nippon Suisan Gakkaishi Bulletin 52, 459- 466.
Acknowledgement
This article was originally published in Life Science Journal, Volume 8, Issue 1, 2011. Engormix.com thanks the author and the journal for this contribution.
Related topics
Authors:
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post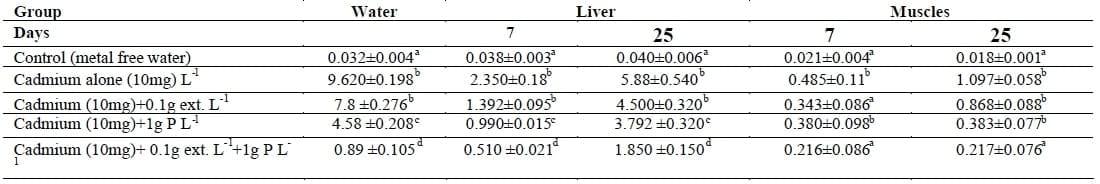



.jpg&w=3840&q=75)