Introduction.
The gastrointestinal tract (GIT) is developmentally very active in the early period posthatch in poultry species (Uni et al., 2000). The intestinal crypts that form on the day of hatch become defined in the first 48 to 96 h and continue to grow rapidly during the first 7 d (Uni et al., 2000). The intestinal villi increase significantly in diameter and length during the first 7 to 10 d after hatching (Denbow, 2000; Sklan, 2001). Subtherapeutic antibiotics have been used in poultry production as an economical means of both increasing growth and preventing disease and are thought to enhance gastrointestinal maturity (Dibner and Richards, 2005). However, the emergence of antibiotic resistance in pathogenic bacteria has led to international reconsideration of the use of antibiotics in animal agriculture (Thwaites and Frost, 1999; Bywater, 2005). Therefore, finding alternative ways to accelerate gastrointestinal maturation of newly hatched birds may be necessary to replace antibiotic growth promoters.
Brewer’s yeast (Saccharomyces cerevisiae) extracts, which are by-products of beer manufacturing, have been added to animal feeds for years for their nutritional content (Westendorf and Wohlt, 2002). Because they also have immunomodulating activity, it is thought that they may serve as alternatives to antibiotics for both growth promotion and disease resistance in poultry production. Whole yeast or yeast cell walls have been shown to improve the growth of both broiler chicks (Zhang et al., 2005) and turkey poults (Bradley et al., 1994). The efficacy of brew er’s dried yeast may be related to its composition, because it is a source of both mannan-oligosaccharides (MOS) and β-glucans.
Table 1. Effect of Alphamune treatment on the ileal morphology of poults at 7 and 21 d of age1.
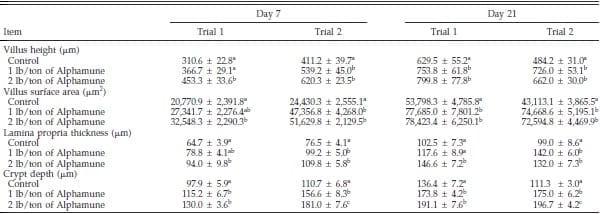 a–c
a–cDifference (P < 0.05) between treatments (vertical).
1Means +- SEM representing 9 birds per group and 20 measurements per parameter per bird.
Mannan-oligosaccharides are polysaccharide-protein complexes derived from yeast that are indigestible to nonruminant animals and can function as prebiotics, providing favorable conditions for beneficial intestinal Lactobacillus spp. (Flickinger and Fahey, 2002). They also provide competitive binding sites for pathogens with mannosespecific fimbriae such as Salmonella, causing them to pass through the intestine, thus decreasing attachment and colonization (Newman, 1994). They have been shown to improve the BW of turkeys grown to market age (Parks et al., 2001; Sims et al., 2004; Zdunczyk et al., 2005) and to improve feed conversion efficiency in turkeys grown to 20 wk (Fritts and Waldroup, 2003).
β-Glucans are polymers of glucose that can also be derived from yeast cell walls, bacteria, fungi, and cereals such as oats, barley, and rye. A β-glucan product was recently shown to decrease Salmonella enterica serovar Enteritidis organ invasion and stimulate phagocytosis, bacterial killing, and oxidative burst in heterophils isolated from 4-d-old male Leghorn chickens 24 h after oral challenge (Lowry et al., 2005). Recent results demonstrated that β-glucans as a feed supplement provided protection from the effects of Escherichia coli challenge in broiler chicks (Huff et al., 2006).
Alphamune is a yeast extract antibiotic alternative containing both MOS and β-glucans. Alphamune has been shown to improve feed conversion and BW, stimulate the immune system, and reduce Salmonella colonization in chickens (Van Immerseel et al., 2000). We hypothesized that the MOS and β-glucans in brewer’s yeast extracts may accelerate maturation of the GIT in young poults, leading to better nutrient absorption and disease protection when used as a feed additive. However, very little information exists on how these additives influence enteric physiology during development. The objective of this study was to evaluate the effect of a brewer’s yeast extract feed additive (Alphamune) that combines both the immunomodulatory properties of a standardized level of β-glucans with the performance enhancement of MOS on the maturation of the GIT of turkey poults.
Materials and methods.
Birds and housing.
All experimental conditions and animal protocols were approved by an institutional animal care and use committee. In replicate trials, male Hybrid Converter turkey poults were obtained from a commercial hatchery at day of hatch. All birds were wing-banded and placed into brooder battery pens. In both trials, birds were kept under incandescent lighting on a light schedule consisting of 23 h light and 1 h dark.
Experimental design.
Two replicate trials were conducted to evaluate gut morphology in young turkey poults 7 and 21 d posthatch. Poults were provided ad libitum access to water and an unmedicated standard corn and soybean turkey starter diet that met or exceeded the NRC (1994) recommended allowances, or the same diet supplemented with 1 lb/ton or 2 lb/ton of a standardized yeast extract feed supplement (n = 18 poults/group, Alphamune, Alpharma Animal Health, Antwerp, Belgium). In each replicate trial, gut morphology was evaluated on d 7 or 21 (n = 9/group per d).
Morphometric analysis of the gut.
For enteric morphometric analysis, birds were euthanized on the designated evaluation day, weighed, and the small intestines collected.A2-cm segment of the midpoint of the duodenum, jejunum, and ileum were removed and fixed in 10% buffered formalin for 72 h. Each segment was embedded in paraffin. A2-_msection of each sample was placed onto a glass slide and stained (see below) for examination with a light microscope (Sakamoto et al., 2000). The gastrointestinal morphometric variables evaluated were villus height, villus surface area, lamina propria thickness, and villus crypt depth as described previously (Solis de los Santos et al., 2005). Morphological parameters were measured using the Image Pro Plus v. 4.5 software package (Media Cybernetics, Silver Spring, MD).
Twenty replicate measurements for each variable studied were measured for each sample. These 20 measurements were then averaged to generate a mean value for each variable for an individual poult. The villus height was measured from the top of the villus to the top of the lamina propria. Surface area was calculated using the formula:
where VW is villus width and VL is villus length (Sakamoto et al., 2000). The lamina propria thickness was measured in the space between the base of the villus and the top of the muscularis mucosa. Crypt depth was measured from the base upward to the region of transition between the crypt and villus (Aptekmann et al., 2001).
Figure 1. Effect of Alphamune treatments of feed (control, cross-hatched bars; 1 lb/ton of Alphamune, open bars; 2 lb/ton of Alphamune, solid bars) on ileum neutral (A), sialomucin (B), and sulfomucin (C) goblet cell density in turkey poults on d 7 and 21. Values are means } SEM representing cell density in 10 well-oriented villi/bird per d of treatment. Means with no common letter differ (P ≤ 0.05) between treatments within trials.
Table 2. Effect of Alphamune treatment on the jejunal morphology of poults at 7 and 21 d of age1.
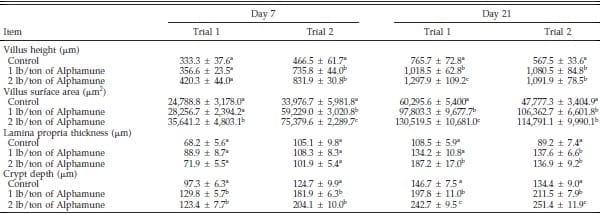 a–c
a–cDifference (P < 0.05) between treatments (vertical).
1Means +- SEM representing 9 birds per group and 20 measurements per parameter per bird.
Differentiation of goblet cells and quantification.
Neutral goblet cells were detected by staining 5-_m sections with periodic acid Schiff staining (PAS) as described previously (McManus, 1948) with slight variations. Following deparaffinization and dehydration, slides were incubated in 0.5% periodic acid for 5 min, washed and incubated with Schiff’s reagent (Sigma Chemical Co., St. Louis,MO)for 20 min, and then counterstained with hematoxylin for 5 min. The number of PASpositive cells staining a red color (PAS+) along the villi was counted by light microscopy as described previously (Uni et al., 2003). To differentiate acidic goblet cells, highiron diamine-Alcian blue stain was used. With the highiron diamine-Alcian blue stain, sialomucins stain blue and sulfomucin goblet cells stain black (Boshuizen et al., 2005). Briefly, after deparaffinization and dehydration, slides were placed in HID solution overnight (16 h), rinsed in water, and then incubated in Alcian blue solution for 5 min (1% in 3% acetic acid, pH 2.5). After being rinsed in water, slides were dehydrated and mounted as described previously (Makkink et al., 2002; Uni et al., 2003). The number of goblet cells per villus was counted after the staining in 10 well-oriented crypt-villus units of the duodenum, jejunum, and ileum, respectively, per bird on d 7 and 21 posthatch.
Statistical analysis.
Gut morphology, BW data, and gut weight data were subjected to ANOVA using SAS (SAS Institute, 2002). Mean separation was accomplished using Duncan’s multiple range tests; P ≤ 0.05 was considered significant.
Results.
Bw
Poults supplemented with Alphamune had increased BW on d 7 (128.7 } 4.5 g, 128.6 } 4.6 g, trial 1; 126.1 } 3.8 g, 131.6 } 1.0 g, trial 2; 1 and 2 lb/ton of Alphamune groups, respectively) compared with control treatments (115.7 } 3.9 g, trial 1; 123.6 } 5.9 g, trial 2, P ≤ 0.05). No differences in BW were observed at d 21. There were no differences in percentage of mortality attributable to treatment in either trial.
Intestinal morphometric parameters.
Gastrointestinal morphology differed by portion of the small intestine and, to some degree, by day of evaluation. At the 2 lb/ton of Alphamune dose, all enteric morphometric characteristics evaluated were higher than those of the control in the ileum (Table 1, Figures 1 and 2). At the lower Alphamune treatment, the numbers of all 3 types of goblet cells in the ileum were higher in both replicate trials than in the control group at d 7 (Figure 1), as was crypt depth (Table 1). Ileum villus height, surface area, and crypt depth were increased on d 21 in both trials in the 1 lb/ton of Alphamune group compared with the control group (Table 1). Laminia propria thickness was consistently higher for the 2 lb/ton of Alphamune treatment groups across days and trials (Table 1).
The jejunum villus surface area and crypt depth were increased in the 2 lb/ton of Alphamune groups compared with the control group for both d 7 and 21 and for the lower dose treatment on d 21 in both trials (Table 2). Jejunum villus height results were inconsistent at d 7 but were greater in both the 1 and 2 lb/ton of Alphamune treatment groups compared with the control group on d 21 (Table 2). Lamina propria thickness was not different on d 7 but was consistently thicker for the 2 lb/ton of Alphamune group compared with the control group on d 21 (Table 2). More sialomucin and sulfomucin goblet cells were observed in the 2 lb/ton of Alphamune treatment compared with the control treatment in both trials on d 7 and 21 in the jejunum (Figure 3). Neutral goblet cell numbers were consistently higher in the jejunum for the Alphamune groups on d 21 only (Figure 3).
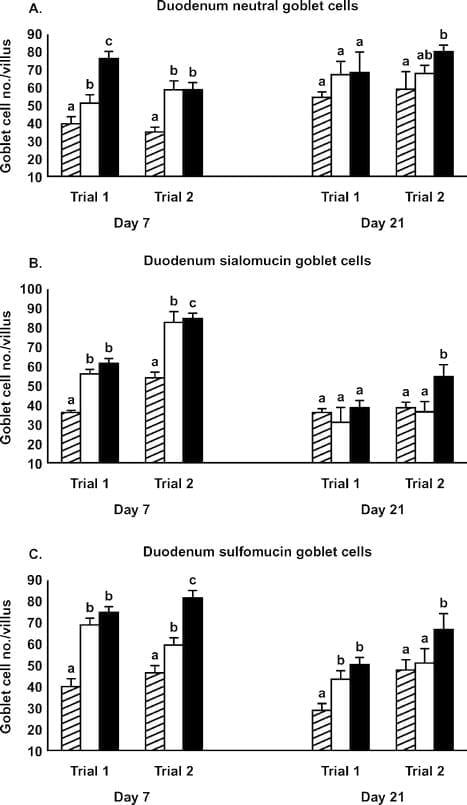
Although some duodenum morphometric parameters differed from controls on d 7, few effects of the Alphamune treatment were observed on d 21 (Table 3). Duodenum villus height and surface area in the 2 lb/ton of Alphamune groups were higher than in the control group on d 7 (Table 3), as were neutral, sialomucin, and sulfomucin goblet cell numbers for both the 1 and 2 lb/ ton of Alphamune treatments for both trials (Figure 4). On d 21, sulfomucin goblet cell densities were higher for the 2 lb/ton of Alphamune treatments across trials (Figure 4).
Yeast extract affects gut morphology in turkey poults
Figure 2. Supplementation of 2 lb/ton of Alphamune in feed increased the number of neutral, acidic (sialomucin and sulfomucin) stained goblet cells in the ileum compared with the control treatment on d 7. A similar effect was observed in the ileum on d 21. Neutral goblet cells stained red (A) with periodic acid Schiff staining. To differentiate acidic goblet cells, a combination of high-iron diamine-Alcian blue (HID-AB) staining was used. With the HID-AB staining, sialomucin goblet cells stained blue (B), whereas sulfated goblet cells stained black (C).
Table 3. Effect of Alphamune treatment on the duodenal morphology of poults at 7 and 21 d of age1.
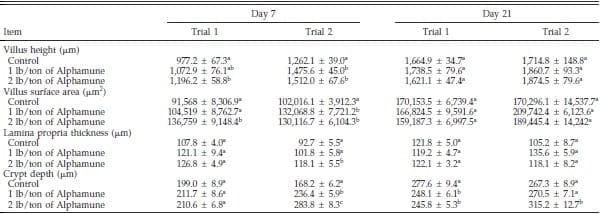 a–c
a–cDifference (P < 0.05) between treatments (vertical).
1Means +- SEM representing 9 birds per group and 20 measurements per parameter per bird.
Discussion
In this study, gastrointestinal maturation was accelerated when poults were fed a brewer’s yeast extract containing β-glucans and MOS. During the first week posthatch, the small intestine develops at a rate that exceeds other body organs (Uni et al., 1998a,b, 1999). This highly active metabolic period and the establishment of microflora in the intestinal tract present a very dynamic environment in the young bird. Multiple studies have demonstrated the ability to accelerate the maturation of the microflora by providing microflora to the neonate (Nurmi and Rantala, 1973; Barnes et al., 1979; Nurmi et al., 1992); however, little research has focused on the gastrointestinal physiology and how nutritional supplements may influence the structure, maturation, and function of the intestine.
Alphamune and other yeast cell components have previously been shown to improve feed conversion and BW in broiler chicks (Zhang et al., 2005) and turkey poults (Bradley et al., 1994; Huff et al., 2006). Early maturation of the gut is directly related to digestion and nutrient absorption in the small intestine, because villi play a crucial role in these processes (Uni et al., 1999; Aptekmann et al., 2001; Gartner and Hiatt, 2001; Sklan, 2001) and may account for the increased BW of poults supplemented with Alphamune at 1 wk of age. Altering the feed and supplementation in neonates can significantly affect enteric development. Villi height is increased by supplementing poultry diets with calcium (Aptekmann et al., 2001), and the lack of feed decreases the number of enterocytes available for villus growth and villus absorptive surface area (Noy et al., 2001). Potturi et al. (2005) report that intestinal villi in turkey poults were significantly increased in birds receiving feed the first week posthatch compared with birds deprived of feed. Additionally, a high-protein diet increases the length of the intestinal villi of the chicken (Yamauchi et al., 1993).
Along with the enhanced nutritional absorption properties of Alphamune, the mannans and β-glucans are proposed to have immunomodulating and disease-resistant properties. In our study, the 2 lb/ton of Alphamune group consistently had significantly enhanced lamina propria thickness, crypt depth, and mucin-producing goblet cells in the ileum, and to some degree in the jejunum and duodenum, compared with the control group. Lamina propria thickness can be used as an indicator of gut health because the lamina propria contains dendritic cells that survey the contents of the lumen and protect against infection by stimulating the adaptive immune response, increasing gut motility, and modifying mucin production, defensin secretion, and IgA production (Macpherson and Harris, 2004). The crypts of the villus contain several specialized cells, including absorptive cells, goblet cells, and regenerative cells, that are responsible for the production of mucus and the replacement of old cells. Because the goblet cells are produced in the crypt (Ayabe et al., 2000), a deeper crypt may reflect the increased density of goblet cells observed in this study.
We observed a pronounced increase in goblet cell numbers with Alphamune supplementation (2 lb/ton group) compared with the controls. This effect was consistent across both d 7 and 21 for the ileum for most goblet cell types in the jejunum, and for d-7 poults in the duodenum. Goblet cells secrete glycoprotein compounds known as mucins (Forstner, 1978), which form the mucus layer that protects the intestinal surface from the invasion of enteric bacteria, bacterial and environmental toxins, and some dietary components that may damage the mucosa (Specian and Oliver, 1991). Mucins are classified into neutral and acidic subtypes; the latter are further distinguished by sulfated (sulfomucin) or nonsulfated (sialomucin) groups (Roberton and Wright., 1997). The physiological relevance of distinct mucin subtypes is not well understood. However, acidic mucins are thought to protect against bacterial translocation in immature birds (Fontaine et al., 1996; Roberton and Wright, 1997).
The mechanism by which Alphamune increases the goblet cell numbers is not clear. Mucin genes are regulated at the transcriptional level by cytokines, bacterial products, and growth factors (Temann et al., 1997). Mucin biosynthesis is also influenced by conditions or agents that affect the differentiation of precursor cells into mature goblet cells and agents or conditions that uncouple the processes of glycosylation and protein synthesis, or which influence protein synthesis (Sharma and Schumacher, 1995; Langhout et al., 1999). Mucin can also serve as a substrate for fermentation by commensal bacteria. Jonsson and coworkers (2001) found that the presence of mucin in the growth medium initiates mucin-binding properties in several strains of Lactobacillus, whereas Gusils and colleagues (2003) demonstrated that Lactobacillus adheres to purified chicken intestinal mucin. Other studies have described mucin as a site for bacterial adhesion (Vimal et al., 2000), with subsequent competition between pathogenic and beneficial bacteria (Craven and Williams, 1998).
Mucin synthesis and secretion are influenced by the diet (Sherman et al., 1985; Sharma and Schumacher, 1995; Uni et al., 2003; Smirnov et al., 2005). Fernandez and coworkers (2000) fed xylanase to chickens, which reduced Campylobacter jejuni cecal colonization by decreasing mucus viscosity, altering gut transit time, and possibly “flushing” C. jejuni from the GIT. Smirnov et al. (2005) observed that goblet cell density was greater in the ileum and jejunum and that mucin glycoprotein levels were lower in the duodenum of chicks fed antibiotic growth promoters. Probiotics increased the goblet cell cup in the lower intestines of chicks in that study. In vitro studies using Lactobacillus plantarum 299v demonstrate the ability of probiotics to inhibit enteropathogenic organisms by inducing intestinal mucin gene expression (Mack et al., 1999). Thus, mucin plays an important role in enteric protection from pathogens.
These results suggest that feed supplemented with Alphamune, an MOS and β-glucan additive, may accelerate gastrointestinal maturation in turkey poults. In addition, the results provide clues to the immunostimulatory effects of this product because the numbers of neutral, sialomucin, and sulfomucin goblet cells in the GIT were increased in supplemented poults.
Yeast extract affects gut morphology in turkey poults
Figure 3. Effect of Alphamune treatments of feed (control, cross-hatched bars; 1 lb/ton of Alphamune, open bars; 2 lb/ton of Alphamune, solid bars) on jejunum neutral (A), sialomucin (B), and sulfomucin (C) goblet cell density in turkey poults on d 7 and 21. Values are means } SEM representing cell density in 10 well-oriented villi/bird per d of treatment. Means with no common letter differ (P ≤ 0.05) between treatments within trials.
Solis de los santos et al.
Figure 4. Effect of Alphamune treatments of feed (control, cross-hatched bars; 1 lb/ton of Alphamune, open bars; 2 lb/ton of Alphamune, solid bars) on duodenum neutral (A), sialomucin (B), and sulfomucin (C) goblet cell density in turkey poults on d 7 and 21. Values are means } SEM representing cell density in 10 well-oriented villi/bird per d of treatment. Means with no common letter differ (P ≤ 0.05) between treatments within trials.
Acknowledgments.
We are grateful to Steven Clark and Kurt Dobson, Alpharma Animal Health, for providing Alphamune for these studies.Wealso gratefully acknowledge the excellent technical assistance of Dana Bassi, Sonia Tsai, Scott Zornes, David Horlick, Wally McDonner, and David Cross.
References.
1. Aptekmann, K. P., S. M. Baraldi Arton, M. A. Stefanini, and M. A. Orsi. 2001. Morphometric analysis of the intestine of domestic quails (Coturnix coturnix japonica) treated with different levels of dietary calcium. Anat. Histol. Embryol. 30:277– 280.
2. Ayabe, T., D. P. Satchell, C. L. Wilson, W. C. Parks, M. E. Selsted, and A. J.Ouelette. 2000. Secretion of microbicidal defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1:113–118.
3. Barnes, E. M., C. S. Impey, and B. J. Stevens. 1979. Factors affecting the incidence and anti-salmonella activity of the anaerobic caecal flora of the young chick. J. Hyg. (Lond.) 82:263–283.
4. Bradley, G. L., T. F. Savage, and K. I. Timm. 1994. The effects of supplementing dietswith Saccharomyces cerevisiae var. boulardii on male poult performance and ileal morphology. Poult. Sci. 73:66–70.
5. Boshuizen, J. A., J. H. Reimerink, A. M. Korteland-val Male, V. J. van Ham, J. Bouma, G. J. Gerwig, M. P. Koopmans, H. A. Buller, J. Dekker, and A. W. Einerhand. 2005. Homeostasis and function of goblet cells during rotavirus infection inmice. Virology 5:210–221.
6. Bywater, R. J. 2005. Identification and surveillance of antimicrobial resistance dissemination in animal production. Poult. Sci. 84:644–648.
7. Craven, S. E., and D. D. Williams. 1998. In vitro attachment of Salmonella typhimurium to chicken cecal mucus: Effect of cations and pretreatment with Lactobacillus spp. isolated from the intestinal tracts of chickens. J. Food Prot. 61:265–271.
8. Denbow, D. M. 2000. Gastrointestinal anatomy and physiology. Pages 299–321 in Sturkie’s Avian Physiology. 5th ed. G. C. Whittow, ed. Academic Press, San Diego, CA.
9. Dibner, J. J., and J.D. Richards. 2005.Antibiotic growth promoters in agriculture: History and mode of action. Poult. Sci. 84:634–643.
10. Fernandez, F., R. Sharma, M. Hinton, and M. R. Bedford. 2000. Diet influences the colonization of Campylobacter jejuni and distribution of mucin carbohydrates in the chick intestinal tract. Cell. Mol. Life Sci. 57:1793–1801.
11. Flickinger, E. A., and G. C. Fahey, Jr. 2002. Pet food and feed applications of inulin, oligofructose, and other oligosaccharides. Brit. J. Nut. 87:S297–S300.
12. Fritts, C. A., and P. W. Waldroup. 2003. Evaluation of Bio-Mos mannan oligosaccharide as a replacement for growth promoting antibiotics in diets for turkeys. Int. J. Poult. Sci. 2:19–22.
13. Fontaine, N., J. C. Meslin, S. Lory, and C. Andrieux. 1996. Intestinal mucin distribution in the germ-free rat and in the heteroxenic rat harbouring a human bacterial flora: Effect of inulin in the diet. Br. J. Nutr. 75:881–892.
14. Forstner, J. T. 1978. Intestinalmucins in health and disease. Digestion 17:234–263.
15. Gartner, L. P., and J. L. Hiatt. 2001. Color Textbook of Histology. 2nd ed. W. B. Saunders, Baltimore, MD.
16. Gusils, C., O. Oppezzo, R. Pizarro, and S. Gonza´lez. 2003. Adhesion of probiotic lactobacilli to chick intestinal mucus. Can. J. Microbiol. 49:472–478.
17. Huff, G. R., W. E. Huff, N. C. Rath, and G. Tellez. 2006. Limited treatment with β-1,3/1,6-glucan improves production values of broiler chickens challenged with Escherichia coli. Poult. Sci. 85:613–618.
18. Jonsson, H., E. Strom, and S. Roos. 2001. Addition ofmucin to the growth medium triggers mucus-binding activity in different strains of Lactobacillus reuteri in vitro. FEMS Microbiol. Lett. 204:19–22.
19. Langhout,D. J., J. B. Schutte, P. V. Van Leeuwen, J.Wiebenga, and S. Tamminga. 1999. Effect of dietary high- and low-methylated citrus pectin on the activity of the ileal microflora and morphology of the small intestinal wall of broiler chicks. Br. Poult. Sci. 40:340–347.
20. Lowry, V. K., M. B. Farnell, P. J. Ferro, C. L. Swaggerty, A. Bahl, and M. H. Kogut. 2005. Purified β-glucan as an abiotic feed additive up-regulates the innate immune response in immature chickens against Salmonella enterica serovar Enteritidis. Int. J. Food Microbiol. 98:309–318.
21. Mack, D. R., S.Michail, S.Wei, L.McDougall, andM.A. Hollingsworth. 1999. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276:G941–G950.
22. Macpherson, A. J., and N. L. Harris. 2004. Interactions between commensal intestinal bacteria and the immune system. Nat. Rev. Immunol. 4:478–485.
23. Makkink, M. K., N. M. Schwerbrock, M. Mahler, J. A. Boshuizen, I. B. Renes, M. Cornberg, H. J. Hedrich, A. W. Einerhand, H. A. Buller, S. Wagner, M. L. Enss, and J. Dekker. 2002. Fate of goblet cells in experimental colitis. Dig. Dis. Sci. 47:2286–2297. McManus, J. F. A. 1948. Histological and histochemical uses of periodic acid. Stain Technol. 23:99.
24. NRC. 1994. Nutrient Requirements of Poultry. 9th rev. ed. National Academy Press, Washington, DC.
25. Newman, K. E. 1994. Mannan-oligosaccharides: Natural polymers with significant impact on the gastrointestinalmicroflora and the immune system. Biotechnology in the feed industry. Pages 167–174 in Proc. Alltech’s 10th Annual Sym. T. P. Lyons and K. A. Jaques, ed. Nottingham University Press, Loughborough, Leics, UK.
26. Noy, Y., A. Geyra, and D. Sklan. 2001. The effect of early feeding on growth and small intestinal development in the post hatch poult. Poult. Sci. 80:912–919.
27. Nurmi, E., L. Nuotio, and C. Schneitz. 1992. The competitive exclusion concept: Development and future. Int. J. Food Microbiol. 15:237–240.
28. Nurmi, E., and M. Rantala. 1973. New aspects of Salmonella infection in broiler production. Nature 241:210–211.
29. Parks, C. W., J. L. Grimes, P. R. Ferket, and A. S. Fairchild. 2001. The effect of mannanoligosaccharides, bambermycins, and virginiamycin on performance of large white male market turkeys. Poult. Sci. 80:718–723.
30. Potturi, P. V. L., J. A. Patterson, and T. J. Applegate. 2005. Effect of delayed placement on intestinal characteristics in turkey poults. Poult. Sci. 84:816–824.
31. Roberton, A. M., and D. P. Wright. 1997. Bacterial glycosulphatases and sulphomucin degradation. Can. J. Gastroenterol. 11:361–366.
32. Sakamoto, K., H. Hirose, A. Onizuka, M. Hayashi, N. Futamura, Y. Kawamura, and T. Ezaki. 2000. Quantitative study of changes in intestinal morphology and mucus gel on total parenteral nutrition in rats. J. Surg. Res. 94:99–106.
33. SAS Institute. 2002. SAS/STATUser’s guide: Release 9.03 Edition. SAS Institute Inc., Cary, NC.
34. Sharma, R., and U. Schumacher. 1995. Morphometric analysis of intestinal mucins under different dietary conditions and gut flora in rats. Dig. Dis. Sci. 40:2532–2539.
35. Sherman, P., J. F. Forstner, N. Roomi, I.Kharti, andG. G. Forstner. 1985. Mucin depletion in the intestine of malnourished rats. Am. J. Physiol. 248:G418–G423.
36. Sims, M. D., K. A. Dawson, K. E. Newman, P. Srping, and D. M. Hooge. 2004. Effects of dietary mannan oligosaccharide, bacitracin methylene disalicylate, or both on the live performance and intestinal microbiology of turkeys. Poult. Sci. 83:1148–1154.
37. Sklan, D. 2001. Development of the digestive tract of poultry. World’s Poult. Sci. J. 57:415–428.
38. Smirnov, A., R. Perez, E. Amit-Romach, D. Salan, and Z. Uni. 2005. Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic growth promoter supplementation. J. Nutr. 135:187–192.
39. Solis de los Santos, F., M. B. Farnell, G. Tellez, J. M. Balog, N. B. Anthony, A. Torres-Rodriguez, S. Higgins, B. M. Hargis, and A.M. Donoghue. 2005. Effect of prebiotic on gut development and ascites incidence of broilers reared in a hypoxic environment. Poult. Sci. 84:1092–1100.
40. Specian, D., and M. Oliver. 1991. Functional biology of intestinal goblet cells. Am. J. Physiol. 260(Cell Physiol. 29):C183–C193.
41. Temann, U. A., B. Prasad, M. W. Gallup, C. Basbaum, S. B. Ho, R.A. Flavell, and J.A. Rankin. 1997.Anovel role formurine IL- 4 in vivo: Induction of MUC5AC gene expression and mucin hypersecretion. Am. J. Respir. Cell Mol. Biol. 16:471–478.
42. Thwaites, R. T., and J. A. Frost. 1999. Drug resistance in Campylobacter jejuni, C. coli and C. lari isolated from humans in northwest England and Wales, 1997. J. Clin. Pathol. 52:812–814.
43. Uni, Z., S. Ganot, and D. Sklan. 1998a. Posthatch development of mucosal function in the broiler small intestine. Poult. Sci. 77:75–82.
44. Uni, Z.,A. Geyra,H. Ben-Hur, andD. Sklan. 2000. Small intestinal development in the young chick: Crypt formation and enterocyte proliferation and migration. Br. Poult. Sci. 41:544–551.
45. Uni, Z., Y. Noy, and D. Sklan. 1999. Posthatch development of small intestinal function in the poult. Poult. Sci. 78:215–222.
46. Uni, Z., R. Platin, and D. Sklan. 1998b. Cell proliferation in chicken intestinal epithelium occurs both in the crypt and along the villus. J. Comp. Physiol. B 168:241–247.
47. Uni, Z., A. Smirnov, and D. Sklan. 2003. Pre- and posthatch development of goblet cells in the broiler small intestine: Effect of delayed access to feed. Poult. Sci. 82:320–327.
48. Van Immerseel, F., J. De Buck, F. Pasmans, L. Bohez, A. Martel, F. Boyen, K. De Gussem, F. Haesebrouck, and R. Ducatelle. 2000. Mannan-oligosaccharides (Alphamune) in chicken feed decrease colonization of Salmonella in chickens early after infection. Ghent University, Merelbeke, Belgium, and Alphalma, Berchem, Belgium.
49. Vimal, D. B., M. Khullar, S. Gupta, and N. K. Ganguly. 2000. Intestinal mucins: The binding sites for Salmonella typhimurium. Mol. Cell. Biochem. 204:107–117.
50. Westendorf, M. L., and J. E. Wohlt. 2002. Brewing by-products: Their use as animal feeds. Vet. Clin. North Am. Food Anim. Pract. 18:233–252.
51. Yamauchi, K., E. Nakamura, and Y. Isshiki. 1993. Development of the intestinal villi associated with the increased epithelial cell mitosis in chickens. Anim. Sci. Technol. 64:340–350.
52. Zdunczyk, Z., J. Juskiewicz, J. Jankowski, E. Biedrzycka, and A. Koncicki. 2005. Metabolic response of the gastrointestinal tract of turkey to diets with different levels of mannan-oligosaccharide. Poult. Sci. 84:903–909.
53. Zhang, A. W., B. D. Lee, S. K. Lee, K. W. Lee, G. H. An, K. B. Song, and C. H. Lee. 2005. Effects of yeast (Saccharomyces cerevisiae) cell components on growth performance, meat quality, and ileal mucosa development of broiler chicks. Poult. Sci. 84:1015–1021.
 a–cDifference (P < 0.05) between treatments (vertical).
a–cDifference (P < 0.05) between treatments (vertical).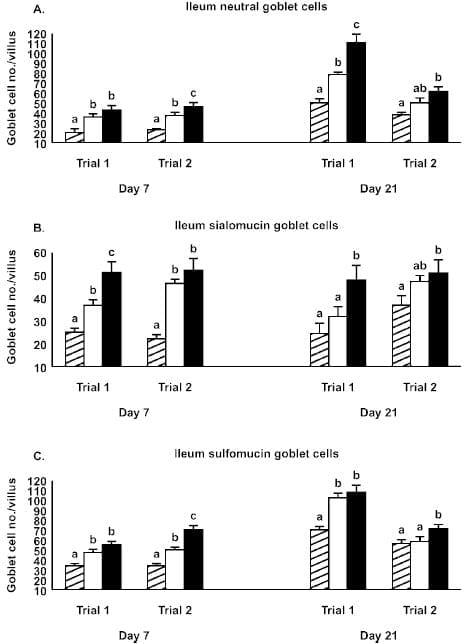
 a–cDifference (P < 0.05) between treatments (vertical).
a–cDifference (P < 0.05) between treatments (vertical).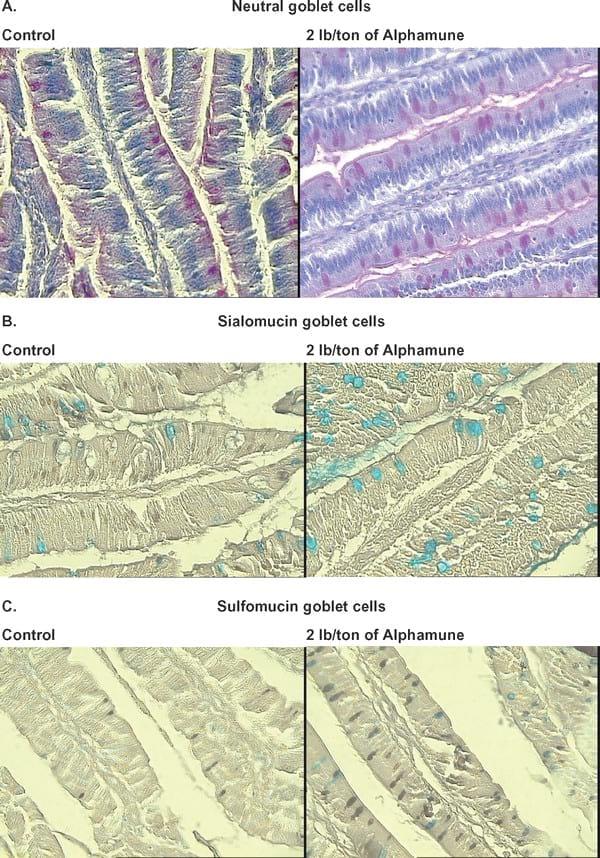
 a–cDifference (P < 0.05) between treatments (vertical).
a–cDifference (P < 0.05) between treatments (vertical).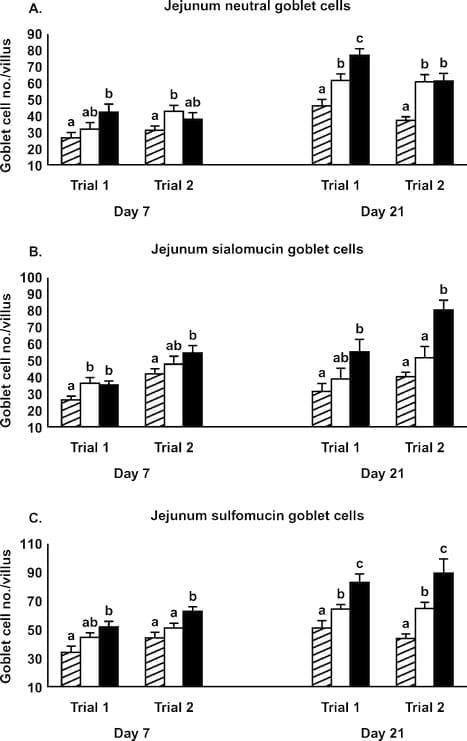








.jpg&w=3840&q=75)