INTRODUCTION
The biogenic amines are biologically active compounds synthesized from amino acids. Feed-borne biogenic amines are most commonly synthesized by spoilage microorganisms and are usually considered to be potential toxins (Eggum et al. 1989). By-products that have undergone some degree of spoilage are generally considered to be the richest sources of biogenic amines. These would include meat and bone meal, blood meal, feather meal, poulty by-product meal and fish meals. The biogenic amines include: gamma-aminobutyric acid, an inhibitory amino acid synthesized from glutamic acid; histamine, a local hormone synthesized from histidine; tyramine, synthesized from tyrosine; cadaverine, synthesized from lysine; serotonin, a neurotransmitter synthesized from tryptophan; dopamine, norepinephrine and epinephrine, neurotransmitters synthesized from tyrosine; and the mammalian polyamines putrescine, spermidine and spermine synthesized from ornithine and methionine.
The identification of feed-borne biogenic amines with toxic syndromes in livestock and poultry has arisen mainly from the presence of histamine. Histamine is a vasodilator and exogenous dietary histamine can promote acid secretion in the stomach and cause ulcers in swine and gizzard erosion in poultry (Harry et al. 1975). Gizzerosine, a thermal decomposition product of histamine and lysine, was identified by Okazaki et al. (1983) as a toxic component of overheated mackerel meal. Gizzerosine has been reported to be a much more potent stimulus of gastric acid secretion in the chicken than histamine and is likely the major cause of gizzard erosion in broilers fed low quality fish meal (Masumura et al. 1985; Hino et al. 1987). Tyramine is a vasoconstrictor and acts to raise blood pressure.
Behavioral modification can arise from elevated brain concentrations of phychoactive biogenic amines. Serotonin acts as a sedative which epinephrine and norepinephrine are stimulatory.
The mammalian polyamines
A subgroup of the biogenic amines are the mammalian polyamines: putrescine, spermidine and spermine. The polyamines are low molecular weight, cationic compounds synthesized from methionine and ornithine in short, highly regulated pathways. The regulatory enzymes are ornithine decarboxylase (ODC) which catalyzes the synthesis of putrescine from ornithine, and S-adenosylmethionine decarboxylase (AdoMetDC) which regulates the donation of aminopropyl groups from methionine to the substrates putrescine and spermidine in the synthesis of spermidine and spermine (Pegg, 1986).
Although the metabolic significance of the mammalian polyamines remains uncertain, it is believed that they are anabolic compounds with hormone-like properties. They contribute to cell homeostasis and are linked to important roles in cell proliferation, differentiation and neoplasia (Seiler, 1992). It has been reported that rapidly growing cells have higher concentrations of polyamines than slowly growing cells. Concentrations of polyamines rise before increases in DNA, RNA and protein synthesis (Morgan, 1990). Cessation of cellular growth has been correlated with reduced concentrations of polyamines (Heby et al. 1990).
Although all body cells have the capacity to synthesize polyamines, active transport mechanisms exist to facilitate intracellular polyamine transport. It has been proposed that exogenous dietary polyamines may play an important role in promoting growth and maintaining human health (Bardocz et al. 1993). The significance of the effect of such polyamines will vary with physiological conditions (Bardocz, 1993). About 10% of dietary putrescine reaches the body pool (Bardocz et al. 1998) and dietary putrescine not required for cellular processes will be catabolized and excreted (Bardocz et al. 1995).
The effect of feed-borne polyamines on growth and metabolism of chicks
The effect of dietary supplements of individual polyamines on chick growth and metabolism has been investigated using purified crystalline amino acid diets. Purified diets were used to minimize the effects of naturally-occurring feed-borne biogenic amines that might confound results. It was observed that chicks fed diets containing 0.2% supplemental putrescine grew significantly faster that controls but 0.8% or greater was toxic (Smith, 1990). Putrescine concentrations were greatly increased in liver, kidney and muscle when supplements were fed. These findings were interpreted as indicating that putrescine may be an essential nutrient for the chick (Anonymous, 1992). This is unlikely, however, as all cells can synthesize putrescine and interorgan transport of the compound has been described.
Spermidine is synthesized from the condensation of putrescine and an aminopropyl group donated by decarboxylated methionine. It has a higher molecular weight and a more positive charge than putrescine and because of this, it can be considered to be more biogenic. The feeding of spermidine to chicks resulted in increased digestive tract development but the potential for increased growth rate was lower than for putrescine. Dietary spermidine supplements were more toxic than putrescine supplements although there were some indications of growth promotion at very low levels (Smith et al., 1996). Spermine is the most highly charged of the mammalian polyamines and has the highest molecular weight. Spermine also proved to be the most toxic of these compounds and had the narrowest potential range for promoting growth (Sousadias and Smith, 1995). It was concluded that the growth promoting potential of the mammalian polyamines declines, while the toxicity increases, with molecular charge and weight.
It is not practical to feed purified polyamines to livestock and poultry because of the cost and complications in feed mixing. Any natural source of these compounds will have as a contaminant the various biogenic amines produced by spoilage microorganisms. One prevalent compound for which there is little toxicity information is cadaverine. Cadaverine is synthesized by bacteria and is found in mammalian cells only due to bacterial synthesis in the intestine or as a dietary contaminant. This polyamine has no known function in mammalian cells and is metabolized and excreted as a xenobiotic compound. Chicks have been shown to be very tolerant to high dietary concentrations of cadaverine (Smith et al. 1996). It was observed, however, that there was enlargement of the intestinal tract in response to increasing dietary concentrations of cadaverine even though this is a microbial metabolite. It must be concluded that polyamine-induced intestinal tract development must be non-specific in nature and is in response to molecular charge. It can also be concluded that cadaverine may occur as a contaminant in feed grade sources of polyamines with no adverse effects on chick performance.
Potential beneficial applications of the feeding of polyamines
Supplementation of practical broiler diets with putrescine failed to promote the growth of birds fed a low protein diet (Colnago and Jensen, 1992). It is likely that potential benefits arising from the feeding of polyamines are most likely when normal functioning of the intestinal tract is inadequate for optimal performance. An example was the report of Grant et al. (1989) that dietary putrescine supplements reduced the adverse effects of soybean-based milk replacers for calves. A similar benefit was seen when neonatal piglets were fed soybean-based milk replacers (Grant et al. 1990).
The effect of dietary putrescine on the toxicity of raw soybeans to chicks
Raw legumes contain anti-nutritional factors including protease inhibitors and lectins. These toxic proteins easily undergo thermal denaturation and the heating process in the production of soybean meal is usually adequate for detoxification. The feeding of raw legumes to poultry and swine results in growth depression due to pancreatic hypertrophy and hypersecretion of digestive enzymes by the exocrine pancreas. Binding of lectins to absorptive surfaces of the intestine reduces nutrient uptake.
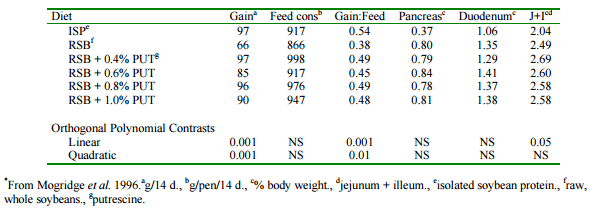
In a series of experiments conducted in our laboratory, chicks were fed diets containing 52% raw, ground, unextracted soybeans (Mogridge et al. 1996). It was observed that supplementing the diet containing raw soybeans with 0.4% putrescine resulted in the same growth rate as was observed for birds fed an isolated soybean protein-based diet (Table 1). Pancreatic hypertrophy induced by the feeding of raw soybeans was not reversed by putrescine supplementation. It was concluded that dietary putrescine was promoting intestinal growth and increasing nutrient absorption. This was counteracting the adverse effects of lectins on nutrient uptake and restoring growth to control levels. The feeding of dietary putrescine could, therefore, allow the feeding of unprocessed legumes as a dietary protein source. This might prove economically advantageous under certain circumstances. A potentially wider application would be to counter the adverse effects of feeding lower quality soybean meals. Such meals arise from inadequate heating of soybeans during processing. This leaves residual protease inhibitiors and lectins which can reduce performance of livestock and poultry.
Table 1. Effect of feeding raw soybeans with and without putrescine on chick growth, feed consumption and organ enlargement.
The effect of dietary putrescine on growth and metabolism of turkeys
High neonatal mortality continues to cause economic losses in several sectors of the livestock and poultry industries including swine and turkey production. Mortality in newly-hatached turkey poults is much greater than that seen in broiler chickens. Commercial turkey poults have a reduced foraging instinct and are slow to seek food and water. The resulting increase in mortality is due to the condition known as “starve out”. This likely contributes to suboptimal development of the digestive tract and serves as a potential application for the feeding of biogenic amines to promote intestinal tract development.
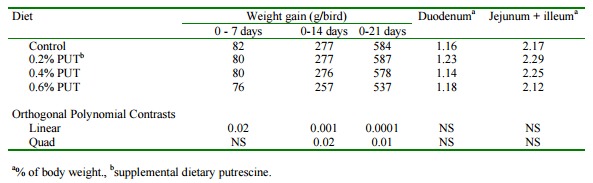
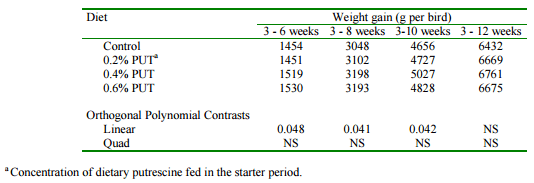
A series of experiments were conducted in our laboratory to determine any potential benefit from the feeding of supplemental dietary putrescine to turkey poults. Hatching eggs were obtained from Hybrid Turkeys, Kitchener, ON. Day-old poults were fed a practical starter diet (28% protein) for three weeks. Diets were supplemented with 0.2, 0.4 and 0.6% purified putrescine and growth and feed consumption were determined weekly. There was a significant curvilinear response to putrescine supplementation with poults fed 0.2% recording the greatest gain (Table 2). There was a substantial accumulation of putrescine in intestinal tissues of birds fed the supplemented diets. There was also an accumulation of putrescine and spermidine in liver and muscle. A subset of birds fed each diet was then fed a conventional, unsupplemented grower diet for the next 9 weeks. A significant carryover effect from the feeding of putescine in the starter period was observed. The heaviest birds were those fed 0.4% putrescine in the starter period (Table 3). It was concluded that putrescine supplements can promote the growth of turkey poults in the starter period and that this precocious development of the intestinal tract results in increased growth rates of birds in the grower phase.
Table 2. Effect of supplemental dietary putrescine on growth and intestinal development in turkey poults.
Table 3. Effect of supplemental dietary putrescine in the starter period on turkey performance in the grower period.
Effect of dietary putrescine on eggshell formation in laying hens
Another application of the feeding of polyamines is a potential improvement in calcium absorption in laying hens. Eggshell quality continues to be an important issue in the egg industry. Putrescine, moreover, has been shown to be a key factor in the mode of action of vitamin D in the chick intestine (Shinki et al. 1991). There is a decline in eggshell quality as hens age and this has been attributed, in part, to reduced intestinal calcium uptake as well as to increased egg size (Al-Batshan et al. 1994).
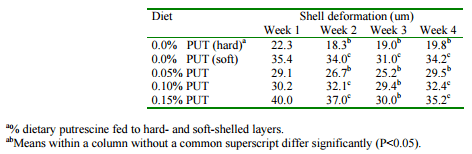
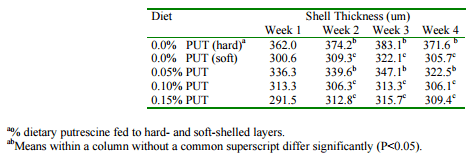
Experiments in our laboratory were conducted, therefore, to determine the potential for dietary supplements of putrescine to influence eggshell quality in laying hens that had been identified as having a tendency to lay soft-shelled eggs. Laying hens were fed a conventional layer diet for 4 weeks during which time egg production and feed consumption were monitored and eggs were collected for determination of egg and shell quality. Diets contained 0, 0.05, 0.10 and 0.15% supplemental putrescine. A group of birds that layed hard-shelled eggs was also included as a control. It was observed that only those birds fed 0.05% supplemental putrescine had no significant increase in the frequency of shell deformations compared to those birds laying hard-shelled eggs (Table 4). The birds fed 0.05% supplemental putresicne were also the only dietary group for which shell thickness was not significantly less than for the hard-shelled controls (Table 5). It can be concluded that when eggshell quality is a concern, there may be economic benefits derived from small supplements of dietary putrescine.
Table 4. Effect of supplemental dietary putrescine on eggshell deformation.
Table 5. Effect of supplemental dietary putrescine on eggshell thickness.
The feeding of polyamines to blue shrimp (Litopenaeus stylirostris)
A series of experiments were conducted at the Universidad Autonoma de Nuevo Leon to determine the effect of feeding practical diets supplemented with purified biogenic amines on growth and metabolism of blue shrimp. Immature shrimp (initial weight 61 - 75 mg) where fed soybean, fish meal and wheatbased diets supplemented with 0, 500, 1100, 2200, 3300 and 4400 mg kg -1 putrescine; 0, 500, 1100, 2200, 3400, and 4500 mg kg-1 spermidine; or 0, 500, 1100, 2300, 3400 and 4600 mg kg-1 spermine. Diets were fed for 28 days with 3 tanks of 10 shrimp per diet. Weight gain, feed consumption and survival rates were determined. At the end of the study, shrimp were lyophilized and pulverized prior to measurement of whole body and hepatopancreas concentrations of biogenic amines by HPLC (TapiaSalazar et al. 2000).
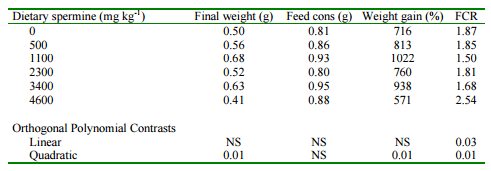
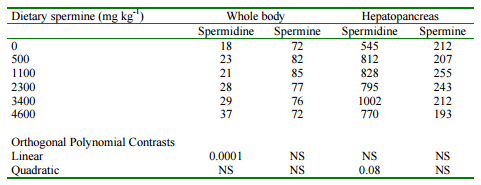
It was found that in contrast to other species, the feeding of supplemental putrescine to shrimp had no effect on growth rate or feed consumption. There was, however, a significant linear increase in spermidine concentrations in both whole body and hepatopancreas with the feeding of increasing concentrations of putrescine. It would appear that the shrimp tissues were metabolizing the flooding doses of putrescine into spermidine. This, however, had no effect on growth. The feeding of spermidine also had no effect on growth rate or feed consumption of shrimp. Tissue concentrations of spermidine increased linearly with the feeding of spermidine in both whole body and hepatopancreas. There was also a trend towards increased spermine in hepatopancreas. It would appear that the shrimp tissues do not metabolize much spermidine to other polyamines. The feeding of increasing supplemental spermine, however, resulted in a significant quadratic effect on final body weight, weight gain and feed conversion ratio (Table 6). The growth promoting effect of spermine was maximized when the supplemental level was 1100 mg kg-1 . It was observed, however, that there was a linear increase in whole body spermidine concentration when supplemental spermine was fed (Table 7). It was concluded that excess dietary spermine is synthesized to spermidine for excretion by the shrimp. The growth promoting effect, however, must be the result of the anabolic effect of spermine because the feeding of spermidine did not result in increased growth rates.
Table 6. Effect of supplemental dietary spermine on growth performance of blue shrimp.
Table 7. Effect of supplemental dietary spermine on tissue polyamine concentrations (ug mg -1) in blue shrimp.
SUMMARY AND CONCLUSIONS
It is now clear that the concept of biogenic amines as only toxic contaminants of feeds is dated. Feedborne mammalian polyamines, particularly putrescine, have the potential to promote growth of numerous species through their biogenic actions at the level of the intestinal tract. This is particularly so when intestinal integrity is compromised as in the feeding of unheated legumes. Other applications would be when intestinal absorptive capacity is suboptimal as with the neonatal piglet, the newly hatched turkey poult and the older laying hen. In the case of shrimp, spermine is the polyamine with the greatest potential to promote growth.
The challenge for the feed industry will be to develop cost effective, polyamine-rich feed supplements that are devoid of harmful biogenic amines such as histamine while maintaining appropriate organoleptic and physical properties.
Presented at the V Simposium Internacional de Nutrición Acuícola, Merida, Yucatán, Mexico.
LITERATURE CITED
Al-Batshan, H.A., Scheideler, S.E., Black, B.L., Garlich, J.D., Anderson, K.E., 1994. Duodenal calcium uptake, femur ash and eggshell quality decline with age and increase following moult. Poultry Sci. 73, 1590-1596.
Anonymous, 1992. Is putrescine an essential nutrient for avians? Nutr. Rev. 50, 81-82.
Bardocz, S., 1993. The role of dietary polyamines. Eur. J. Clin. Nutr. 47, 683-690.
Bardocz, S., Duguid, T.J., Brown, D.S., Grant, G., Pusztai, A., White A., Ralph, A., 1995. The importance of dietary polyamines in cell regeneration and growth. Br. J. Nutr. 73, 819-828.
Bardocz, S., Grant, G., Brown, D.S., Ralph A., Pusztai, A., 1993. Polyamines in food - implications for growth and health. J. Nutr. Biochem. 4, 66-71.
Bardocz, S., Hughes, E.L., Grant, G., Brown, D.S., Duguid, T.J., Pusztai, A., 1998. Uptake, inter-organ distribution and metabolism of dietary putrescine in the rat. J. Nutr. Biochem. 9, 332-338.
Colnago, G.L., Jensen, L.S., 1992. Putrescine effects on performance of male broiler chicks fed low-protein diets supplemented with essential amino acids. Poultry Sci. 71, 211-214.
Eggum, B.O., Hansen, N.E., Sorensen, H, 1989. Amino acid precursors of biogenic amines. In: Friedman, M. (Ed.), Absorption And Utilization Of Amino Acids, Vol. III, CRC Press, Inc., Boca Raton, FL. pp. 67-90.
Grant, A.L., Holland, R.E., Thomas, J.W., King K.J., Liesman, J.S., 1989. The effects of dietary amines on the small intestine in calves fed soybean protein. J. Nutr. 119, 1034-1041.
Grant, A.L., Thomas, J.W., King, K.J., Liesman, J.S., 1990. Effects of dietary amines on small intestinal variables in neonatal pigs fed soy protein isolate. J. Anim. Sci. 68, 363-371.
Harry, E.G., Tucker J.F., Laursen-Jones, A.P., 1975. The role of histamine and fish meal in the incidence of gizzard erosion and proventricular abnormalities in the fowl. Br. Poult. Sci. 16, 69-78.
Heby, O., Holm, I., Persson, L., 1990. Polyamine-mediated control of ornithine decarboxylase and S-adenosylmethionine decarboxylase expression in mammalian cells. Biochem. Soc. Trans. 18, 1084-1087.
Hino, T., Noguchi, T., Naito, H., 1987. Effect of gizzerosine on acid secretion by isolated mucosal cell of chicken proventriculus. Poultry Sci. 66, 548-551.
Masumura, T., Sugahara, M., Noguchi, T., Mori, K., Naito, H., 1985. The effect of gizzerosine, a recently discovered compound in overheated fish meal, on the gastric acid sectretion in the chicken. Poultry Sci. 64, 356-361.
Mogridge, J.L., Smith, T.K., Sousadias, M.G., 1996. Effect of feeding raw soybeans on polyamine metabolism in chicks and the therapeutic effect of exogenous putrescine. J. Anim. Sci. 74, 1897-1904.
Morgan, D.M.L. 1990. Polyamines and cellular regulation: perspectives. Biochem. Soc. Trans. 18, 1080-1084.
Okazaki, T., Noguchi, T., Igarashi, K., Sakagami, Y., Seto, H., More, K., Naito, H., Masumura, T., Sugahara, H., 1983. Gizzerosine, a new toxic substance in fish meal, causes severe gizzard erosion in chicks. Agr. Biol. Chem. 47, 2949-2953.
Pegg, E.A., 1986. Recent advances in biochemistry of polyamines in eukaryotes. Biochem. J. 234, 249-262.
Seiler, N., 1992. The role of polyamines in cell biology. In: Bittar, E.E.(Ed.), Chemistry Of The Living Cell. JAI Press, Greenwich, CT.
Shinki, T., Tanada, H., Takito, J., Yamaguchi, A., Nakamura, Y., Yoshiki, S., Suda, T., 1991. Putrescine is involved in the Vitamin D action in chick intestine. Gastroenterology 100, 113-122.
Smith, T.K., 1990. Effect of dietary putrescine on whole body growth and polyamine metabolism. Proc. Soc. Exper. Biol. Med. 194, 332-336.
Smith, T.K., Fleming, H.E., Seddon, I.R., 1996. The effect of dietary cadaverine on chick growth and intestinal polyamine metabolism. FASEB J. 10, A512 (abstract).
Smith, T.K., Mogridge, J.L., Sousadias, M.G., 1996. Growth-promoting potential and toxicity of spermidine, a polyamine and biogenic amine found in foods and feedstuffs. J. Agric. Food Chem. 44, 518-521.
Sousadias, M.G., Smith, T.K., 1995. Toxicity and growth-promoting potential of spermine when fed to chicks. J. Anim. Sci. 73, 2375-2381.
Tapia-Salazar, M., Smith, T.K., Harris, A., 2000. High-performance liquid chromatographic method for determination of biogenic amines in feedstuffs, complete feeds, and animal tissues. J. Agr. Food Chem. 48, 1708-1712.









.jpg&w=3840&q=75)