Nutritional routes to protection against mycotoxins
Nutritional approaches to reduce the impact of mycotoxins
Mycotoxins are highly toxic secondary products of the metabolism of some fungi mainly belonging to Aspergillus, Penicillium and Fusarium species. A toxic syndrome caused by mycotoxin ingestion in man and animals is referred to as a mycotoxicosis. It has been estimated that at least 300 fungal metabolites are potentially toxic for man and animals, and that as much as 25% of the world’s cereals are contaminated with known mycotoxins (Devegowda et al., 1998).
Moreover, it can be realistically assumed that other mycotoxins are likely to be discovered. The most notorious and extensively investigated mycotoxins are aflatoxin B1, ochratoxin A, fumonisin B1, zearalenone, deoxynivalenol and T2-toxin. However, recently research interest in other toxins such as citrinin, sterigmatocistyn and diacetoxyscirpenol has been growing.
Chemical, biological and toxicological properties of mycotoxins are diverse. Hence, their toxic effects are extremely variable and also depend on toxin intake, duration of exposure, animal species, age, sex, physiological status, and eventual synergism among mycotoxins simultaneously present in feed or foods.
However, the main toxic effects are carcinogenicity, genotoxicity, teratogenicity, nephrotoxicity, hepatotoxicity, reproductive disorders and immunosuppression. In addition, some mycotoxins are specifically indicated or strongly suspected to cause certain human and animal diseases such as Reye’s disease (caused by aflatoxin B1) (Becroft and Webster, 1972), equine leukoencephalomalacia and porcine pulmonary edema (caused by fumonisin B1), human alimentary toxic aleukia (caused by T2-toxin) and Balkan endemic nephropathy (caused by ochratoxin). The positive correlation between the consumption of aflatoxin-contaminated foods and the increased incidence of liver cancer in several Asian and African populations has led to the classification of aflatoxins as group 1A carcinogens by the International Agency for Research on Cancer (IARC, 1993).
In 1997, the Joint FAO/WHO Expert Committee on Food Additives (JECFA) provided qualitative and quantitative information on aflatoxins and concluded that aflatoxins should be treated as carcinogenic food contaminants, the intake of which should be reduced to levels as low as could reasonably be acheived (JECFA, 1997). According to Kuiper-Goodman (1995), although human health risk assessment involves toxicological, epidemiological and exposure factors, in the risk management of mycotoxins it is necessary to take action before all this information is available. However, the lack of this information means some uncertainties in assessing human exposure and health risk and in establishing causal relationships between incidence of mycotoxins in foods and human disease (Smith et al., 1995).
From a regulatory standpoint, different countries have enforced different thresholds to limit the passage of mycotoxins along the food chain. In the US it is required by law that aflatoxin M1 in milk be less that 0.5 ppb, whereas in western Europe the regulations are more stringent, and maximum levels are set at 0.05 ppb (Boutrif and Canet, 1998). Currently the US Food and Drug Administration (FDA) regulates only aflatoxin among the mycotoxins and stipulates a maximum of 20 ppb of aflatoxin in grain shipped for interstate transit. Denmark, on the other hand, has decided that a level of 15 ppb of ochratoxin in the liver or kidney of pigs results in confiscation of those organs and that levels exceeding 25 ppb would result in condemnation of the entire carcass.
No doubt exists concerning the economic impact of mycotoxins. Recent studies (Charmley et al., 1995; Garcia et al., 1997) evidenced that economic losses occur at all levels of food and feed production including crop and animal production, processing and distribution. Even during favorable climatic periods millions of dollars are lost as a consequence of crop contamination. For all these reasons prevention, decontamination and detoxification of mycotoxins are issues of great importance.
Generally, any approach aimed to reduce the toxic and economic impact of mycotoxins should fulfill the following prerequisites:
1. prevent, destroy, remove or detoxify mycotoxins in feeds and foods
2. not produce nor leave toxic and/or carcinogenic/mutagenic residues in the final products
3. not significantly alter important technologic and nutritional properties
4. be technically and economically feasible (Piva et al., 1995)
A wide range of chemical, physical and biological routes have been taken in the attempt to reduce the toxicity of mycotoxins. Although some chemical detoxification methods (i.e., ammonia, sodium bisulfite and calcium hydroxide treatments) are effective, they do not fulfill all the requirements, especially those concerning the safety of reaction products and the safeguarding of the nutritional characteristics of the treated foods and feeds (Piva et al., 1995).
For these reasons nutritional approaches such as a) supplementation of nutrients, food components or additives with protective properties against toxicity, and b) addition of non-nutritive sorbents or bacteria, yeast and modified yeast cells capable of reducing mycotoxin bioavailability are assuming increasing interest.
Nutritional routes to protection against mycotoxins
ANTIOXIDANT SUBSTANCES
Since some mycotoxins are known to cause membrane damage through increased lipid peroxidation, the protective properties of antioxidant substances have been extensively investigated. Selenium, some vitamins (A, C and E) and their precursors have marked antioxidant properties acting as superoxide anion scavengers. For these reasons they have been investigated as protective agents against mycotoxins.
Selenium
In a controlled in vitro study Lin et al. (1994) observed that selenium could reduce toxicity of T-2 toxin on cultured chicken embryonic chondrocytes. When sodium selenite was added to the culture in the presence of T-2 toxin there were no decreases in collagen microfibril, intramembrane particle numbers and enzyme (cytochrome c, oxidase and H+-ATPase) activities observed.
In an in vivo study in rats Shi et al. (1994) demonstrated that selenium inhibits aflatoxin B1-DNA binding and adduct formation. Sodium selenite and a selenium-enriched yeast extract protected cultured hamster ovary cells from aflatoxin B1 cytotoxicity, but not from mutagenicity (Shi et al., 1995).
In contrast, McLeod et al. (1997) reported that rats given a seleniumdeficient diet were more resistant to aflatoxin B1 than those given a selenium-sufficient diet. According to the authors the protection conferred by selenium deficiency was associated with the hepatic expression of an aldoketo reductase and a glutathione S-transferase subunit that efficiently metabolize the mycotoxin.
Based on a study on rats, Atroshi et al. (1995) concluded that selenium, vitamin E and vitamin C act as an antioxidant and free radical scavenger system that protects the spleen and brain against membrane damage caused by T-2 toxin and deoxynivalenol. Additionally, it has been postulated that higher levels of methionine supplementation would counteract the methionine depletion due to the fact that glutathione is composed of methionine and cystine (Devegowda et al., 1998).
Vitamins
Further evidence of protective effects of some vitamins and/or their precursors against mycotoxin-induced damage arises from numerous in vivo and in vitro studies. Grosse et al. (1997) observed that vitamin A, C and E reduced DNA adducts in kidney and liver by 70 to 90% in mice exposed to ochratoxin and zearalenone. Vitamin C can also protect guinea pigs from aflatoxin B1 hepatotoxicity (Netke et al., 1997). Vitamin C reduced abnormalities in both mitotic and meiotic chromosomes and morphologies of the sperm head in mice exposed to ochratoxin (Bose and Sinha, 1994).
Analogous protective actions have also been attributed to vitamin E (Ibeh and Saxena, 1998) and vitamin A against exposure both to ochratoxin (Kumari and Sinha, 1994) and aflatoxin B1 (Sinha and Dharmshila, 1994).
Supplementary vitamin E administered to chickens partially counteracts the formation of lipid peroxides due to single and combined exposure to ochratoxin and T-2 toxin (Hoehler and Marquardt, 1996) while Coelho (1996) demonstrated that vitamin supplementation of turkey diets can reduce negative effects of mycotoxins and environmental stress.
Carotenoids (carotene and xanthophylls) are excellent antioxidants with antimutagenic and anticarcinogenic properties. They occur naturally in some foods such as carrots, red tomatoes, butter, cheese, paprika, palm oil, corn kernels, and red salmon. Dietary carotenoids inhibit aflatoxin B1-induced liver DNA damage in rats as demonstrated by Gradelet et al. (1997; 1998).
To the authors, ß-apo-8'carotenal, canthaxanthin and astaxanthin exert their protective effects altering aflatoxin B1 metabolism toward pathways that lead to the formation of aflatoxin M1, a less toxic metabolite. As specifically regards betacarotene, because it does not alter aflatoxin B1 metabolism its protective action must be mediated by other mechanisms. No protective effects were observed by administration of supplementary lycopene (a carotenoid found in ripe fruit) and excess vitamin A.
In a study on antimutagenic activity of natural xanthophylls against aflatoxin B1 in Salmonella typhimurium, Gonza´lez de Mejı´a et al. (1997) reported that xanthophylls inhibited the mutagenicity of aflatoxin B1 in a dose-dependent manner.
Yu et al. (1994), using woodchuck hepatocytes as a model to investigate the effects of vitamin A, C, E and betacarotene on aflatoxin B1-DNA adducts, reported contrasting results. In fact, they showed that vitamin C and particularly vitamin A were effective in reducing aflatoxin B1-DNA binding, whereas vitamin E and betacarotene enhanced it. The authors concluded that additional studies are needed to understand the mechanism of enhanced adduct formation.
Two vitamin A2 compounds (3-dehydroretinol and 3-dehydroretinyl palmitate) mainly present in freshwater fish have been shown to be very effective in inhibiting the microsome-catalyzed formation of DNA-aflatoxin B1 adduct (Aboobaker et al., 1997). The inhibition should be due to modulation of microsomal enzymes, which activate the carcinogen, hence suggesting a potential chemopreventive role of these compounds against carcinogenesis induced by aflatoxin B1.
In a study conducted on Bacillus subtilis cells, vitamin E was able to prevent the genotoxicity of zearalenone (Ghe´dira Che´kir et al., 1998). The authors attributed the specificity of the prevention to the structural similarity of vitamin E and zearalenone.
FOOD COMPONENTS AND ADDITIVES
Numerous food components, ingredients or additives with or without overall antioxidant properties have been investigated to verify their chemoprotective properties. Ellagic acid is a phenolic compound that occurs naturally in some foods such as strawberries, raspberries, and grapes. It has both antimutagenic and anticarcinogenic activity as demonstrated in a wide range of assays in vitro and in vivo.
Loarca Pina et al. (1996; 1998), in in vitro tests on salmonella cells, showed that ellagic acid inhibits aflatoxin B1 direct-acting mutagenicity, particularly when incubated with metabolic enzymes. The result of sequential incubation indicated that the formation of an aflatoxin B1-ellagic acid chemical complex was the mechanism of inhibition.
Another study emphasized the role of phenolic compounds in the activation and detoxification processes and hence in modulating the carcinogenicity of aflatoxin B1 (Aboobaker et al., 1994). In tests performed on rats given a synthetic diet containing various food-associated phenolic compounds each at 0.5% level, the authors observed a marked decrease in the ability of liver microsomes to catalyze reactions of aflatoxin B1 leading to its activation and DNA adduct formation.
The phenolic compounds tested were several flavonoids (fisetin, kaempferol, morin, naringin and catechin), phenolic acids (caffeic acid and chlorogenic acid), other phenolic (eugenol, vanillin) and synthetic phenolic antioxidants butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT). Some phenolic compounds (naringin, catechin, eugenol, vanillin and BHA) were also found to induce cytosolic glutathione S-transferase activity that stimulated the formation of specific aflatoxin B1-glutathione conjugate.
Williams and Iatropoulos (1996) and Manson et al. (1997) confirmed that BHA and BHT inhibited the initiation of hepatocarcinogenesis by aflatoxin B1 in rats. Soni et al. (1997) confirmed that ellagic acid and BHA ameliorate aflatoxin-induced mutagenicity and carcinogenicity. The same authors also found that certain food additives and/or active ingredients with general antioxidant properties such as turmeric (Curcuma longa), curcumin (diferlolyl methane) and garlic, have the same properties. Firozi et al. (1996) demonstrated that curcumin reduces the formation of aflatoxin B1-DNA adducts by modulating cytochrome P450 function.
A range of natural dietary constituents including garlic oil, ethoxyquine, indole-3-carbinol and phenethyl isothiocynate have in vitro chemoprotective actions toward aflatoxin B1 (Manson et al., 1997). Some natural polyphenolic compounds, i.e., polyhydroxylated flavonoids and phenolic acids, were found to be effective in reducing aflatoxin B1-DNA adducts (Firozi and Bhattacharya, 1995). S-methyl methanethiosulfonate (MMTS), a compound present in the juice of cabbage and onion, has a suppressive effect on aflatoxin B1- or methyl methanesulfonate (MMS)-induced chromosome aberrations in rat bone marrow cells (Ito et al., 1997). Also, the precursor of MMTS S-methyl-L-cysteinesulfoxide significantly suppressed aflatoxin B1- or MMS-induced chromosome aberrations.
According to the authors, although other mechanisms are not excluded, the protective properties of MMTS may result from its ability to modify -SH groups in proteins.
Cavin et al. (1998), in a study on the effects of two diterpenes (cafestol and kahweol) present in green and roasted coffee beans against the covalent binding of aflatoxin B1 metabolites to DNA of rats, identified these two substances as potentially chemoprotective agents. It has been postulated that these compounds may act as blocking agents by producing a coordinated modulation of multiple enzymes involved in carcinogen detoxification. Manson et al. (1997) found that caffeic acid has chemoprotective activity against aflatoxin B1 in vitro.
Jesval (1998) attributed to berry and leaf juice of grape (Vitis vinifera) protective abilities against ochratoxin-induced hepatoma and renal carcinoma inmice. Propionic acid and potassium sorbate used as preservatives in bread making in France destroyed aflatoxin B1 from 52 to 71% (Amra et al., 1996).
ASPARTAME
Three studies (Creppy et al., 1995; 1996; Baudrimont et al., 1997a,b) reported that aspartame (L-aspartyl-L-phenylanine methyl ester) has a wide protective action against ochratoxin-induced subchronic effects.
Studies on monkey kidney cells showed that aspartame prevents or partially protects against some typical cytotoxic effects of ochratoxin such as inhibition of protein synthesis, lipid peroxidation and leakage of certain enzymes such as lactate dehydrogenase, gamma-glutamyl transferase and alkaline phosphatase. In vitro, aspartame prevented ochratoxin binding to plasma proteins.When given to rats, aspartame prevented ochratoxin genotoxicity and nephrotoxicity and washed out the toxin from the body efficiently.
The protective action is thought to be due to its structural similarity to phenylanine and ochratoxin. To the authors, aspartame is the best candidate for preventing ochratoxin-induced subchronic effects, and the absence of side effects in humans and animals is worth noting.
PIPERINE
According to Reen et al. (1997), piperine (1-piperoylpiperidine), the major alkaloid constituent of pepper (Piper nigrum), has considerable potential as protective agent against the carcinogenic effects of aflatoxin B1. It is well known that aflatoxin B1 toxicity is bioactivated by the cytochrome P450 monooxygenases (CYP450). In cultured rat cells piperine dramatically reduced CYP4502B1 activity and counteracted CYP4502B1- mediated toxicity of aflatoxin B1, thus offering a chemopreventive effect against procarginogens activated by CYP4502B1.
COUMARIN
Coumarin (1,2-benzopyrone), a natural food constituent especially present in Fava tonka, has a chemoprotective action against aflatoxin B1. As demonstrated by Goeger et al. (1998) in in vitro studies on hamster ovary cells, liver cells from rats and chick embryos, coumarin decreased cytotoxicity and mutagenicity of aflatoxin B1, with marked species differences in chemoprotection. However, it must be considered that coumarin has also toxic properties and, due to their structural similarity, counteracts vitamin K absorption.
CHLOROPHYLL AND ITS DERIVATIVES
Dashwood et al. (1998) demonstrated that chlorophylline (a food grade derivative of the green plant pigment chlorophyll) has chemopreventive properties against a wide classes of mutagens, including aflatoxin B1. Both in vitro and in vivo studies showed that chlorophylline act as an interceptor molecule by forming a strong noncovalent complex with aflatoxin B1 (Breinholt et al., 1995) reducing hepatic aflatoxin B1-DNA adducts and liver tumors.
CYPROHEPTADINE
Cyproheptadine, a serotonin antagonist with appetite stimulant properties, has been tested to reduce feed refusal due to the presence of deoxynivalenol (Prelusky et al., 1997). The authors observed that cyproheptadine effectively offset the reduction of feed intake. It was concluded that, although serotoninergic mechanisms are involved in reducing deoxynivalenol-induced feed refusal, further investigations are needed to better understand the reasons for the anorectic effect.
MYCOTOXIN BINDING AGENTS
Addition of nutritionally inert sorbents is one of the most recent approaches to reduce mycotoxin effects in animals. After a general initial skepticism, the interest of researchers in sorbents has increased in the last years (Dale, 1998). Sorbents act to reduce the bioavailability of mycotoxins by adsorption. Indeed, if a stable sorbent-mycotoxin complex is formed, the absorption of mycotoxins in the gastrointestinal tract can be reduced thereby decreasing both toxic effects for animal and carry over in animal products for human consumption.
With this aim numerous sorbents of different sources have been tested including hydrated sodium calcium aluminosilicate (HSCAS), zeolites, bentonites, clays and activated carbons (Piva et al., 1995; Ramos et al., 1996).
HSCAS
HSCAS, a phyllosilicate derived from natural zeolite, is perhaps the most extensively investigated sorbent. Evidence of a high affinity of HSCAS for aflatoxin B1 both in vitro and in vivo arises from numerous studies reviewed by Piva et al. (1995) andRamos et al. (1996). However, enthusiasm for the efficacy of HSCAS must be tempered by other studies that have shown HSCAS to be ineffective in binding dangerous mycotoxins other than aflatoxin B1. Indeed, its protective properties are very low toward ochratoxin and zearalenone and nil toward trichothecenes.
Zeolites
Zeolites are hydrated aluminosilicates of alkali and alkaline-earth cations characterized by infinite three-dimensional structures (Ramos et al., 1996).
Although contrasting results are present in the literature, an overall efficacy of zeolite in binding aflatoxin B1 and zearalenone has been reported (Piva et al., 1995; Ramos et al., 1996). As evidenced by Piva et al. (1995), the origin of zeolite can markedly affect results of adsorption tests. In fact, the pore size distribution of synthetic as opposed to natural zeolites varies very little.
If pore size is compatible with that of the mycotoxin molecule, adsorption can occur. On the contrary, adsorption can be low or nil due to the absence of intermediate sized pores. The use of a zeolite, the clinoptilolite, reduced liver accumulation when administered to laying hens exposed to aflatoxin B1 although it had no effect on liver mixed-functionoxygenase (MFO) activities (Zaghini et al., 1998).
Bentonites
Bentonites are sorbents with a layered (lamellar) crystalline microstructure and variable composition and adsorption properties, mainly depending on the interchangeable cations (Na+, K+, Ca+2 and Mg+2) present in the layers (Ramos et al., 1996).
Bentonite has been shown to bind aflatoxin B1 in vitro and reduce its toxic effects in trout and pigs and also to reduce toxic effects of T2-toxin in rats (Ramos et al., 1996). Sodium bentonite and a synthetic zeolite mixture (80:20 ratio) did not depress feed intake or nutrient apparent digestibility (Rizzi et al., 1995) while preventing aflatoxin accumulation in the liver of growing lambs and decreasing aflatoxin recovery in urine by several fold (Zaghini et al., 1993).
To the authors, the detoxifying properties of bentonite could be enhanced by its ability to reduce the transit time of digestion through the gastrointestinal tract thus increasing the fecal loss of the toxins. However, this ability was not observed for zearalenone and nivalenol (Ramos et al., 1996).
Clays
Other clays such as kaolin, sepiolite and montmorillonite have a variable ability to reduce toxic effects of aflatoxin B1 as reviewed by Ramos et al. (1996). However, their efficacy is limited to aflatoxin B1 and is lower than that of HSCAS and bentonite.
Activated carbons
Activated carbons are an important group of sorbents. They are a family of carbonaceous substances obtained by pyrolysis of several organic compounds and manufactured by activation processes aimed at developing a highly porous structure (Galvano et al., 1996a).
Generally, the adsorption properties of activated carbon are strictly dependent on the source materials and physicochemical parameters such as surface area and pore size distribution. As such, preparation methods and chemical treatments can strongly modify activated carbon surface characteristics. Because of the numerous possible combinations between typology of carbonaceous substances and activation processes, many activated carbons with quite different adsorbing properties exist. This fact could explain some contrasting results reported in a recent review concerning the ability of activated carbon to bind mycotoxins (Ramos et al., 1996).
As a reliable universal test of adsorptive properties does not exist for activated carbons, they must be selected under application conditions.
However, in characterizing activated carbons several physicochemical parameters can be considered (Galvano et al., 1996a). Indeed, it is known that the adsorption properties are roughly correlated with the total surface area measured by adsorption of a very small molecule (nitrogen).
The pore size distribution of activated carbon is another important characteristic affecting the accessibility of the internal carbon surface.As diffusion effects inside the pores can slow down the adsorption process, the effective pore size distribution of activated carbon can influence it as a function of the molecular size of the adsorbate. Information about mesopores and macropores can be obtained by mercury porosimetry, which allows determination of the size distribution of pores of inside diameter greater than 75 Ar . Iodine number is a relative indicator of activated carbon microporosity and is often used as an approximation of surface area.
Methylene blue index is a test which establishes the medium size pore (mesopores) range and is an important indicator in practice of activated carbon ability to adsorb organic molecules of medium-large size from a solution (Galvano et al., 1996a).
In a series of preliminary in vitro tests we investigated the adsorption ability of 19 experimental activated carbons from different source materials (i.e., spent olive residues, peach stones and almond shells) obtained with laboratory equipment by several experimental activation processes appropriately selected to obtain the desired physicochemical parameters.
In addition, we investigated four commercial activated carbons produced in industrial processing equipment.
An overall evidence of the high ability of activated carbon in binding mycotoxins in vitro was observed in our studies (Galvano et al., 1996a,b 1997, 1998). The highest abilities were noted in the adsorption of aflatoxin B1 and ochratoxin, whereas the lowest were seen in the adsorption of deoxynivalenol (Table 1). In addition, activated carbons have been shown to efficiently adsorb fumonisin B1 and aflatoxin B1simultaneously.When compared to HSCAS, activated carbons showed much higher adsorption abilities toward all the tested mycotoxins. Thus, activated carbons are capable of binding several mycotoxins in vitro and it is reasonable to consider their potential use as multi-mycotoxin sequestering agents.
Table 1. In vitro adsorption (saturation limits) of mycotoxins from standard solutions by activated carbons or activated carbons or (HSCAS) (μg of mycotoxin/g of sorbent).1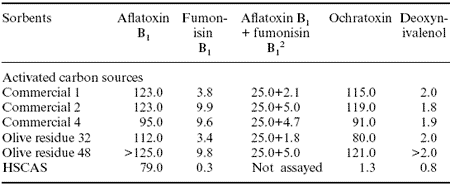
1Galvano et al., 1998.
2Simultaneously.
The molecular size and physicochemical properties of the mycotoxins clearly affect binding efficiency. For this reason further studies on the binding mechanism such as chemisorption indices are needed to clarify and improve adsorption.
Following the preliminary tests we performed two studies intended to verify in vivo the binding ability of one of the activated carbons that had the highest potential in in vitro adsorption. In an experiment on dairy cows we compared the abilities of activated carbon and HSCAS in reducing carryover of aflatoxin B1 from feed to milk.
Activated carbon reduced carryover up to 50%, whereas HSCAS reduction of carryover was 36% (Galvano et al., 1996b). In rats given a fumonisin-contaminated diet toxin bioavailability was indirectly monitored by measuring the sphinganine concentration and the sphinganine/sphingosine ratio in urine, liver and kidney. The addition of 2% activated carbon prevented the increase in liver weight, reduced the sphinganine concentration in liver and the sphinganine concentration and sphinganine/sphingosine ratio in kidneys (Solfrizzo et al., 1998).
Even though some of these responses only approached statistically significance, the data are promising and further studies are in progress in our laboratory. In any case, that is the first report of an in vivo protective action of a sorbent toward fumonisin B1. Although the tests are strongly encouraging, we are extremely conscious that further in vivo studies are required to confirm the efficacy of activated carbon in preventing or reducing the toxic effects of mycotoxins. We assume that the in vivo efficacy of activated carbon will be lower, since the practical conditions differ widely from the experimental ones. More specifically, the binding sites of activated carbon can be occupied by many other compounds present in the feed.
Assuming that in vivo studies would confirm the effectiveness of detoxifying mycotoxins, three questions on whether activated carbon could be added to feeds must be answered. The first is the possible longterm undesired adsorption of essential nutrients such as vitamins and minerals. If long-term in vivo studies should confirm it, two strategies could be adopted: 1) addition of supplemental essential nutrients demonstrated to be excessively adsorbed by activated carbon, or 2) increase the selectivity of activated carbon toward mycotoxins by modulating the activation process and the physicochemical properties.
The second question, as indicated by Ramos et al. (1996), is related to the property of activated carbon to blacken the environment, the animals and the feed. Some manufactures have overcome this problem by producing activated carbon containing up to 65% water with the consistency of brown sugar, thus eliminating the problems associated with the use of the powder form.
We suggested that some of these problems can be also eliminated by pelleting the feed. Furthermore, we observed that, at least for aflatoxin B1, pelleting can increase the sorbent efficacy (Galvano et al., 1996b). The third question is the economic viability of adding activated carbon to feed. Today the price of activated carbon is perhaps prohibitive to the feed industry. However, the possibility of using activated carbon in feed should increase the demand and bring about a practical price for this market. In any case, evaluation of the cost/benefit balance is needed.
Cholestyramine
Cholestyramine, a bile acid-binding resin, was tested as protective agent against ochratoxin-induced nephrotoxicity in rats (Kerkadi et al., 1998).
Cholestyramine decreased the concentration of ochratoxin A in plasma and toxin/metabolite excretion in urine and bile and increased excretion in feces. These results agree with those of Madyastha et al. (1992). The authors attributed the decrease in nephrotoxicity to the reduction of ochratoxin A bioavailability and/or enterohepatic circulation.
Cholestyramine also bound zearalenone (Trenholm et al., 1996). However, the authors noted that its high cost would make its commercial use economically prohibitive.
Polyvinylpolypyrrolideoxynivalenole
Polyvinylpolypyrrolideoxynivalenole (PVPP), a synthetic resin, was shown to bind aflatoxin B1 in feed (Thalib, 1995). The author noted that 0.4 g/kg PVPP can bind up to 50 μg/kg aflatoxin in feed.
Bovine serum albumin
Hirano et al. (1994) demonstrated that bovine serum albumin provided protection against aflatoxin B1 toxic effects. In studies on day-old chicks the authors observed a marked reduction in histological and biochemical symptoms of exposure to aflatoxin B1 and of the toxin level in the plasma and liver. The authors concluded that bovine serum albumin is able to bind aflatoxin B1 in the intestinal tract and is excreted with it. The binding mechanism occurring between bovine serum albumin and aflatoxin B1 was highlighted by Vyjaynthi et al. (1995).
Biological binding agents
Increasing interest has also been generated in using biological products to reduce the bioavailability of mycotoxins to farm animals, particularly as these overcome the inherent drawbacks associated with inorganic sorbents. Saccharomyces cerevisiae 1026, used primarily as an aid to rumen fermentation, was found to have beneficial effects on weight gain and immune response in broilers exposed to aflatoxin B1 (Devegowda et al., 1998).
In vitro studies showed an aflatoxin dose-dependent binding capacity of S. cerevisiae up to 77% (Devegowda et al., 1996). Interestingly, modified mannanoligosaccharide (Mycosorb, Alltech Inc.) derived from the cell wall of S. cerevisiae was reported to have even higher binding capacity (95% aflatoxin, 80% zearalenone, fumonisin up to 59%, and vomitoxin up to 12%; Devegowda et al., 1998). This was further confirmed by the addition of 0.11% modified mannanoligosaccharide to the diet of layers receiving 2.5 ppm of aflatoxin B1 (Figure 1). Aflatoxin did not contaminate the egg, but a 46% decrease in aflatoxin level in the liver was observed (4.13 vs 2.21 ppb; Rizzi, personal communication).
The transformation of mycotoxins upon fermentation has been reported a number of times. Deoxynivalenol and zearalenone were degraded in vitro by the normal bacterial gut flora from the distal sections (cecum, colon, and rectum) of the gastrointestinal tract of pigs (Kollarczik et al., 1994), whereas microorganisms from the proximal segments showed no transformation activity.
Deoxynivalenol was de-epoxidated and zearalenone was hydrolyzed toa-zearalenol and an unknown metabolite. These observations appear to be confined to the type of mycotoxin considered. Incubation with up to 1000 mM fumonisin B1 had no effect on growth rate of various Gram-positive and Gram-negative bacteria nor was fumonisin concentration in the media altered (Becker et al., 1997). Flavobacterium aurantiacum was the only microorganism that removed aflatoxin from liquid medium and food products in significant amounts without the production of toxic by-products (Lillehoj et al., 1967; Line and Brackett, 1995).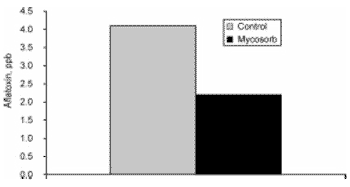
Figure 1. Effect of Mycosorb (0.11% of the diet), a modified mannan oligosaccharide, on liver aflatoxin content of layers receiving 2.5ppm of aflatoxin B1.
More recently, some dairy strains of lactic acid bacteria were found capable of removing aflatoxin B1 from contaminated liquid media via a rapid process involving the removal of approximately 80% of aflatoxin B1 immediately upon contact without further incubation (El-Nezami et al., 1998a).
Heat-treated bacteria had the same ability to remove aflatoxin B1 as viable bacteria, consequently metabolic degradation by viable bacteria has been ruled out as a possible mode of action under the experimental conditions tested. All the Gram-positive strains tested were more efficient than Escherichia coli suggesting that the ability of bacteria to remove aflatoxin B1 is dependent on cell wall structure. The response was dependent on temperature and bacterial concentration, whereas no difference was observed due to pH variation across the range of pH 4 to 6.
Furthermore, treatment with hydrochloric acid, autoclaving or boiling enhanced the binding activity of the bacterial pellets (El-Nezami et al., 1998b), confirming that the type and structure of the cell wall is crucial for an effective binding of mycotoxins.
Megharaj et al. (1997) demonstrated the ability of a mixed culture of bacteria to remove zearalenone from culture media while Pseudomonas aeruginosa was able to inhibit the growth of Penicillium citrinum and the production of citrinin (Giridhar and Reddy, 1997).
| Conclusion Encouraging results have been obtained in studies on the protective action of a large number of nutrients, food components and additives. Generally, compounds with marked antioxidant properties seem to be potentially very efficacious. However, most of the studies focus on the most known mycotoxins, i.e., aflatoxin B1 and ochratoxin, whereas much less information is available concerning more recently discovered mycotoxins such as fumonisins. Moreover, most of the results are based on in vitro studies. Thus, a great deal of work is needed to establish the biochemical mechanisms of action, verify the response in vivo and to search for protective compounds against a broader range of mycotoxins. As regards mycotoxin binding agents evaluated in our laboratory, activated carbon seems to have the higher potential as a multi-toxin binding agent. Though more research is needed on activated carbons and other sequestering agents, nutritional strategies to reduce the toxic and economic impact of mycotoxins seem to be the most promising approach. |
References
Authors: ANDREA PIVA1 and FABIO GALVANO2Aboobaker, V.S., A.D. Balgi and R.K. Bhattacharya. 1994. In vivo effect of dietary factors on the molecular action of aflatoxin B1: role of non-nutrient phenolic compounds on the catalytic activity of liver fractions. In vivo 8:1095-1098.
Aboobaker, V.S., N. Sarma, U.C. Goswami and R.K. Bhattacharya. 1997. Inhibition of microsomal activation of aflatoxin B1 by 3-dehydroretinol and 3-dehydroretinyl palmitate. Indian J. Exp. Biol. 35:1125-1127.
Amra, H.A., S.A.Z. Mahmoud, A.H. Taha and M.A. El-Azab. 1996. Destruction of aflatoxin B1 and G1 in bread making. Mycotox. Res. 12:73-78.
Atroshi, F., A. Rizzo, I. Biese, M. Salonen, L.A. Lindberg and H. Saloniemi. 1995. Effects of feeding T-2 toxin and deoxynivalenol on DNA and GSH contents of brain and spleen of rats supplemented with vitamin E and C and selenium combination. J. Anim. Phys. Anim.Nutr. 74:157-164.
Baudrimont, I., R. Ahouandjivo and E.E. Creppy. 1997a. Prevention of lipid peroxidation induced by ochratoxin A in Vero cells in culture by several agents. Chem. Biol. Interact. 104:29-40.
Baudrimont, I., A.M. Betbeder and E.E. Creppy. 1997b. Reduction of the ochratoxin A-induced cytotoxicity in Vero cells by aspartame. Arch. Toxicol., 71(5)290-298.
Becker, B., H. Bresch, U. Schillinger and P.G. Thiel. 1997. The effect of fumonisin B1 on the growth of bacteria. World Journal of Microbiology and Biotechnology, 13:539-543.
Becroft, D.M.W. and D.R.Webster. 1972. Aflatoxins and Reye’s disease. Br. Med. J. 4:117.
Bose, S. and S.P. Sinha. 1994. Modulation of ochratoxin-produced genotoxicity in mice by vitamin C. Food Chem. Toxicol. 32:533-537.20
Boutrif, E. and C. Canet. 1998. Mycotoxin prevention and control: FAO programmes. Revue Med. Vet. 149: 681-694
Breinholt, V., M. Schimerlik, R. Dashwood and G. Bailey. 1995. Mechanisms of chlorophyllin anticarcinogenesis against aflatoxin B1 complex formation with the carcinogen. Chem. Res. Toxicol. 8:506-514.
Cavin, C., D. Holzhauser, A. Constable, A.C. Huggett and B. Schilter. 1998. The coffee-specific diterpenes cafestol and kahweol protect against aflatoxin B1-induced genotoxicity through a dual mechanism. Carcinogenesis 19:1369-1375.
Charmley L.L., H.L. Threnholm, D.B. Prelusky and A. Rosemburg. 1995. Economic losses and decontamination. Nat. Toxins 3:199-203.
Coelho, M. 1996. Optimum vitamin supplementation needed for turkey performance and profitability. Feedstuffs 68:13-21.20
Creppy, E.E., Baudrimont I. and A.M. Betbeder. 1995. Prevention of nephrotoxicity of ochratoxin A, a food contaminant. Toxicol. Lett. 71:869-877.
Creppy, E.E., I. Baudrimont, A. Belmadani and A.M. Betbeder. 1996. Aspartame as a preventive agent of chronic toxic effects of ochratoxin A in experimental animals. Food Addit. Contam. 13:51-52. 20
Dale, N.Mycotoxin binders: It’s time for real science. 1998. Poult. Digest. 57:38-39.
Dashwood, R., T. Negishi, H. Hayatsu, V. Breinholt, J. Hendricks and G. Bailey. 1998. Chemopreventive properties of chlorophylls toward aflatoxin B1:a review of the antimutagenicity and anticarcinogenicity data in rainbow trout. Mutat. Res. 399:245-514.
Devegowda, G., Aravind B.I.R and M.G. Morton. 1996. Saccharomyces cerevisiae and mannanoligosaccharides to counteract aflatoxicosis in broilers. Proc. Australian Poult. Sci. Symp., Sydney, Australia 8:103-106.
Devegowda, G., Raju M.V.L.N. and H.V.L.N. Swamy. 1998. Mycotoxins: novel solutions for their counteraction. Feedstuffs, December 7, 1998; 12-15.
El-Nezami, H., P.Kankaanpaa, S. Salminen and J. Ahokas. 1998a. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen, aflatoxin B1. Food and Chemical Toxicology, 36:321-326.
El-Nezami,20H., Kankaanpaa, P., Salminen, S. and J. Ahokas. 1998b. Physicochemical alterations enhance the ability of dairy strains of lactic acid bacteria to remove aflatoxin from contaminated media. J. Food Prot. 61:466-468.
Firozi, P.F. and R.K. Bhattacharya. 1995. Effects of natural polyphenols on aflatoxin B1 activation in a reconstituted microsomal monooxygenase system. J. Biochem. Toxicol. 10:25-31.
Firozi, P.F., V.S. Aboobaker and R.K. Bhattacharya. 1996. Action of curcumin on the cytochrome P450-system catalyzing the activation of aflatoxin B1. Chem. Biol. Interact. 100:41-51.
Galvano, F., A. Pietri, B. Fallico, T. Bertuzzi, S. Scire, M. Galvano and R. Maggiore. 1996a. Activated carbons: in vitro affinity for Aflatoxin B1 and relation of adsorption ability to physicochemical parameters. J. Food Prot. 59:545-550.20
Galvano, F., A. Pietri, T. Bertuzzi, G. Fusconi, M. Galvano, A. Piva and G. Piva. 1996b. Reduction of carryover of aflatoxin from cow feed to milk by addition of activated carbons. J. Food Prot. 59:551-554.20
Galvano, F., A. Pietri, T. Bertuzzi, M. Bognanno, A. De Angelis, L. Chies and M. Galvano. 1997. Activated carbons: in vitro affinity for fumonisin B1 and relation of adsorption ability to physicochemical parameters. J. Food Prot. 60:985-991.20
Galvano, F., A. Pietri, T. Bertuzzi, A. De Angelis, A. Piva, L. Chies and M. Galvano. 1998. Activated carbons: in vitro affinity for ochratoxin A and deoxynivalenol and relation of adsorption ability to physicochemical parameters. J. Food Prot. 61:469-475.
Garcia R.P., C.L. Padilla, M. sidik, B.M.Rejesus,R.P.Garcia, B.R. Champ, M. Bengston, O.S. Dharmaputa and H. Halid. 1997. Mycotoxins and its significance in the implementation of general agreement on tariff and trade (GATT). Proceedings of the symposium on20pest management for stored food and feed. Bogor, Indonesia, 5-7 September 1995. BIOTROP Special Publication 59:33-51.
Ghedira Chekir, L, K. Maaroufi, A. Zakhama, F. Ellouz, S. Dhouib, E.E. Creppy and H. Bacha. 1998. Induction of a SOS repair system in lysogenic bacteria by zearalenone and its prevention by vitamin E. Chem. Biol. Interact. 113:15-25.
Giridhar P. and S.M. Reddy. 1997. Efficacy of some bacteria in the control of growth and citrinin production by Penicillium citrinum. Indian Phytopathology 50:1-5.
Goeger, D.E., K.E. Anderson and A.W. Hsie. 1998. Coumarin chemoprotection against aflatoxin B1-inducedmutation in a mammalian cell system: a species difference in mutagen activation and protection with chick embryo and rat liver S9. Environ. Mol. Mutagen. 32:64-74.
Gonzalez de Mejıa, E., M. Ramos Gomez and G. Loarca Pina. 1997. Antimutagenic activity of natural xanthophylls against aflatoxin B1 in Salmonella typhimurium20. Environ. Mol. Mutagen. 30:346-53.
Gradelet, S., P. Astorg, A.M. Le Bon, R. Berges and M. Suschetet. 1997. Modulation of aflatoxin B1 carcinogenicity, genotoxicity and metabolism in rat liver by dietary carotenoids: evidence for a protective effect of CYP1A inducers. Cancer Lett. 114:221-223.20
Gradelet, S., A.M. Le Bon, R. Berges,M. Suschetet and P. Astorg. 1998. Dietary carotenoids inhibit aflatoxin B1-induced liver preneoplastic foci and DNA damage in the rat: role of the modulation of aflatoxin B1 metabolism. Carcinogenesis 19: 403-411.20
Grosse, Y., L. Chekir Ghedira, A. Huc, S. Obrecht Pflumio, G. Dirheimer, H. Bacha and A. Pfohl Leszkowicz. 1997. Retinol, ascorbic acid and alpha-tocopherol prevent DNA adduct formation in mice treated with the mycotoxins ochratoxin A and zearalenone. Cancer Lett. 114: 225-229.20
Hirano, K., Y. Adachi and S. Ishibashi. 1994. Possible role of bovine serum albumin for the prevention of aflatoxin B1-adsorption from the intestinal tract in young chicks. J. Vet. Med. Sci. 56:281-286.
Hoehler, D. and R.R. Marquardt. 1996. Influence of vitamins E and C on the toxic effects of ochratoxin A and T-2 toxin in chicks. Poult. Sci. 75:1508-1515.20
Ibeh, I.N. and D.K. Saxena. 1998. Effect of a-tocopherol supplementation on the impact of aflatoxin B1 on the testes of rats. Exp. Toxicol. Pathol. 50:221-224.
International Agency forResearch on Cancer (IARC). 1993. Some naturally occurring substances: food items and constituent, heterocyclic aromatic amines and mycotoxins. IARC Monographs on the evaluation of carcinogenic risks to humans, vol. 56. IARC, Lyon.
Ito, Y., Y. Nakamura, and Y. Nakamura. 1997. Suppression of aflatoxin B1- or methyl methanesulfonate-induced chromosome aberrations in rat bone marrow cells after treatment with S-methyl methanethiosulfonate. Mutat. Res. 393:307-316.
JECFA. 1997. Toxicological evaluation of certain food additives. WHO Feed Additives Series. 49th meeting of JECFA, Geneva, 1997.
Jesval P. 1998. Antidotal effect of grape juice (Vitis vinifera) on ochratoxin A caused hepatorenal carcinogenesis in mice (Mus musculus). Cytobios 93:123-128.
Kerkadi A., C. Barriault, B. Tuchweber, A.A. FrohlichR.R. Marquardt, G. Bouchardand and I.M. Yousef. 1998. Dietary cholestyramine reduces ochratoxin A-induced nephrotoxicity in the rat by decreasing plasma levels and enhancing fecal excretion of the toxin. J. Toxicol. Environ.Health 3:231-250.
Kollarczik, B., M. Gareis and M. Hanelt. 1994. In vitro transformation of the Fusarium mycotoxins deoxynivalenol and zearalenone by the normal gut microflora of pigs. Natural Toxins 2:3 105-110.
Kuiper-Goodman, T. 1995. Mycotoxins: risk assessment and legislation. Toxicol. Lett. 82-83:853-859.
Kumari, D. and S.P. Sinha. 1994. Effect of retinol on ochratoxinproduced genotoxicity in mice. Food Chem. Toxicol. 32:471-475.
Lillehoj, E.B., A. Ciegler and H.H. Hall. 1967. Aflatoxin B1 uptake by Flavobacterium aurantiacum and resulting toxic effects. J. Bacteriol. 93: 464-471
Lin, Z.H., S.G. Li, L.Y.Wu, S. Sun and Q.W. Lu. 1994. Antagonistic effect of Se on the T-2 toxin-induced changes in the ultrastructure and mitochondrial function of cultured chicken embryonic chondrocytes. J. Clinical Biochemistry Nutrition 17:119-132.
Line, J.E. and R.E. Brackett. 1995. Factors affecting aflatoxin B1 removal by Flavobacterium aurantiacum. J. Food Prot. 58: 91-94.
Loarca Pina, G., P.A. Kuzmicky, E. Gonzalez de Mejıa, N.Y. Kado and D.P. Hsieh. 1996. Antimutagenicity of ellagic acid against aflatoxin B1 in the Salmonella microsuspension assay. Mutat. Res. 360:15-21.
Loarca Pina, G., P.A. Kuzmicky, E.G. de Mejıa and N.Y. Kado. 1998. Inhibitory effects of ellagic acid on the direct-acting mutagenicity of aflatoxin B1 in the Salmonella microsuspension assay. Mutat. Res. 398:183-187.
Madhyastha, M.S., A.A Frohlich. and R.R. Marquardt. 1992. Effects of dietary cholestyramine on the elimination pattern of ochratoxin A in rats. Food Chem. Toxicol. 30:709.
Manson, M.M., H.W. Ball, M.C. Barrett, H.L. Clark, D.J. Judah, G. Williamson and G.E. Neal. 1997. Mechanism of action of dietary chemoprotective agents in rat liver: induction of phase I and II drug metabolizing enzymes and aflatoxin metabolism. Carcinogenesis 18:1729-1738.
McLeod, R., E.M. Ellis, J.R. Arthur,G.E. Neal,D.J. Judah, M.M. Manson, J.D. Hayes. 1997. Cancer-Research-Baltimore 57:4257-4266.20
Megharaj M., I. Garthwaite and JH. Thiele. 1997. Total biodegradation of the oestrogenic mycotoxin zearalenone by a bacterial culture. Lett. Appl. Microbiol. 24:329-333.
Netke, S.P., M.W. Roomi, C. Tsao and A. Niedzwiecki. 1997. Ascorbic acid protects guinea pigs from acute aflatoxin toxicity. Toxicol. Appl. Pharmacol. 143:429-435.20
Piva, G., F. Galvano, A. Pietri and A. Piva. 1995. Detoxification methods of aflatoxins. A review. Nutr. Res. 5:689-715.
Prelusky,D.B., and B.A.Rotter, B.K. Thompson and H.L. Trenholm. 1997. Effect of the appetite stimulant cyproheptadine on deoxynivalenolinduced reductions in feed consumption and weight gain in the mouse. J. Environmental Science and Health 32: 429-448.
Ramos, A.J., J. Fink-Gremmels and E. Hernandez. 1996. Prevention of toxic effects of mycotoxins by means of nonnutritive adsorbent compounds. J. Food Prot. 59:631-641.
Reen, R.K., F.J. Wiebel and J. Singh. 1997. Piperine inhibits aflatoxin B1-induced cytotoxicity and genotoxicity in V79 Chinese hamster cells genetically engineered to express rat cytochrome P4502B1. J. Ethnopharmacology 58:165-173.
Rizzi, L., L. Lambertini, L. Marchesini and A. Zaghini. 1995. Affinity sorbent of aluminosilicate for aflatoxin B1: effects on digestibility in growing lambs. Proceedings of 47 Congresso Nazionale SISVet, 1329-1333.
Shi, C.Y., S.C. Chua, H.P. Lee and C.N. Ong. 1994. Inhibition of aflatoxin B1-DNA binding and adduct formation by selenium in rats. Cancer Lett. 82:203-208.20
Shi, C.Y.,Y.C.Hew and C.N. Ong. 1995. Inhibition of aflatoxin B1-induced cell injury by selenium: an in vitro study. Hum. Exp. Toxicol. 14:55-60.20
Sinha, S.P. and K. Darmshila. 1994. VitaminAameliorates the genotoxicity in mice of aflatoxin B1-containing Aspergillus flavus infested food. Cytobios 79:85-95.
Smith, J.E., G. Solomons, C. Lewis and J.G. Anderson. 1995. Role of mycotoxins in human and animal nutrition and health. Nat. Toxins. 3:187-192.
Solfrizzo,M., G. Avantaggiato,M.R. Carratu, F. Galvano, A. Pietri and A. Visconti. 1998. The use of biomarkers to assess the in vivo effect of activated carbon on fumonisins fed through diets contaminated with Fusarium moniliforme. Revue Med. Vet. 149:667.
Soni, K.B., M. Lahiri, P. Chackradeo, S.V. Bhide and R. Kuttan. 1997. Protective effect of food additives on aflatoxin-induced mutagenicity and hepatocarcinogenicity. Cancer Lett. 115:129-133.
Thalib, A. 1995. Detoxification of aflatoxin in feed with a binder of polyvinylpolypyrrolideoxynivalenole. Jurnal Ilmiah Penelitian Ternak Klepu. (Indeoxynivalenolesia) 1:43-48.
Trenholm, H.L., L.L. Charmley, K.L. Underhill and D.B. Prelusky. 1996. Reducing toxicity of mycotoxins in feed. In: Proceedings of the 4th International Feed Production Conference. February 22 -23 Piacenza, Italy. pp. 69-86.
Vyjaynthi, V., A.K. Capoor and R.B. Sashidhar. 1995. Binding characteristics of bovine serum albumin-aflatoxin B1 to polystyrene microtiter plates: importance of hapten to carrier protein molar ratio. Indian J. Exp. Biol. 33:329-332.
Williams, G.M. and M.J. Iatropoulos. 1996. Inhibition of the hepatocarcinogenicity of aflatoxin B1 in rats by low levels of the phenolic antioxidants butylated hydroxyanisole and butylated hydroxytoluene. Cancer Lett. 104:49-53.
Yu, M.W., Y.J. Zhang, W.S. Blaner and R.M. Santella. 1994. Influence of vitamins A, C, and E and beta-carotene on aflatoxin B1 binding to DNA in woodchuck hepatocytes. Cancer 73:596-604.
Zaghini, A., L. Lambertini, L. Rizzi and P. Roncada. 1993. Effects of a bentonite supplemented diet on prevention of aflatoxicosis in growing lambs. Proceedings of 47 Congresso Nazionale SISVet. 1329-1333.
Zaghini, A., P. Roncada, P. Anfossi and L. Rizzi. 1998. Aflatoxin B1 oral administration to laying hens: effects on hepatic MFO activities and efficacy of a zeolite to prevent aflatoxicosis B1. In: Proceedings of Mycotoxins in the Food Chain –20 Processing and toxicological aspects, a satellite symposium of the 8th International Congress of Toxicology, July 2-4, 1998. Toulouse France.
1 Dipartimento di Morfofisiologia Veterinaria e Produzioni Animali, Universita di Bologna, Ozzano Emilia, Bologna, Italy.
2 Dipartimento di Scienze e Tecnologie Agroforestali ed Ambientali, Universita di Reggio Calabria, Reggio Calabria, Italy.






.jpg&w=3840&q=75)