Mycotoxins in the human food chain
Mycotoxins in the human food chain: what risks for the consumer?
Mycotoxins are highly undesired substances that should not be present in food and for which a zero tolerance is ideal. However, even the best agricultural, storage and processing practices cannot completely avoid or eliminate these contaminants, and thus it is impossible to achieve a truly mycotoxin-free food chain. Despite low consumer awareness of the problem, health risk related to mycotoxin ingestion has been quantified as exceeding risk from other food-related contaminants such as pesticides, additives, heavy metals, and microbial agents. For this reason mycotoxins have been called the ‘hidden killers’.
Mycotoxins can easily enter the human food chain directly via plant products (e.g., cereal grains, oilseeds, nuts, coffee, cocoa), fruits and their juices, beverages (wine and beer), spices and, indirectly, via foods obtained from animals given diets contaminated with mycotoxins that can leave residues in milk and its derivatives, and, especially, in fresh and cured pork. A review of the occurrence of mycotoxins in the most important human foods and the associated risks is the objective of this paper.
Human breast milk
Humans who ingest mycotoxin-contaminated foods eliminate variable amounts of the toxin in bodily fluids or accumulate them in tissues. For this reason, contamination of human breast milk presents a potentially serious health hazard. The occurrence of aflatoxin in human tissues or fluids is a problem, particularly in tropical or subtropical countries. Aflatoxin M1 (AFM1) has been detected in breast milk, cord blood, and maternal blood in African countries (Sudan, Ghana, Kenya, Nigeria, Sudan, Gambia), in the Guangxi region of China, (see review by Galvano et al., 1996), in the United Arab Emirates (Abdulrazzaq et al., 2003), and in Australia and Thailand (el Nezami et al., 1995). Aflatoxin contamination also poses a serious prenatal health hazard because it can cross the human placental membrane and may be concentrated by the developing fetoplacental unit. Although the interaction between dietary aflatoxin intake and exposure of mother, fetus, and newborn infant is very complex, depending on the physiological status of the mother and on food composition, the presence of aflatoxin B1 (AFB1)and its metabolites in human blood and breast milk presents a serious health hazard.
Just as AFB1 is a concern in tropical or subtropical countries, conversely ochratoxin A (OTA) residues in human milk are common in countries in the temperate and cold areas of the northern hemisphere, such as Italy (Micco et al., 1991; 1995; Miraglia et al., 1995), Switzerland (Zimmerli and Dick, 1995), Sweden (Breitholtz-Emanuelsson et al., 1993), and Germany (Gareis et al., 1988). Nevertheless, Jonsyn and coworkers (1995) reported that, due to the high OTA levels in human milk, infants in Sierra Leone are exposed to OTA and AFB1 levels that in some cases far exceed those permissible in animal feed in developed countries.
In Norway, Skaug and co-workers (1998, 2001) examined the relationship between OTA contamination of human milk and dietary intake, finding that the risk of OTA contamination was related to dietary intake of breakfast cereals, processed meat products, cheese, liver pate, cakes, cookies and juice. Because in the first months of life infants may be fed concurrently breast and dried milk sources, they are at risk for ingesting multiple mycotoxins (e.g., OTA plus AFM1).
Animal-derived foods
MILK AND MILK PRODUCTS
The occurrence of mycotoxins in milk and its derivatives is a serious problem of food hygiene, as milk is a primary source of human nutrition, particularly for infants and children, who are potentially more sensitive to toxins and whose diet is much less varied than that of adults.
Major concerns about milk contamination by mycotoxins are largely limited to AFB1 and OTA.
Mammals who ingest AFB1-contaminated diets eliminate into milk amounts of the main hepatic 4-hydroxylated metabolite known as ‘milk toxin’ or aflatoxin M1 (AFM1). Aflatoxin M1 is usually considered to be a detoxification product of AFB1 as its toxicity is about one order of magnitude less than that of AFB1; however, the International Agency for Research on Cancer (IARC, 1993) classifies AFM1 as a possible human carcinogen.
OTA transfer to milk has been demonstrated in rats and humans. Dietary OTA can also result in residues in cow’s milk but at substantially lower levels except when massive doses are ingested. Indeed, OTA is largely transformed by the rumen microflora into ochratoxin-α. However, Skaug (1999) found that OTA levels in milk from cows in Norway were sufficient to cause a higher intake of OTA than the suggested tolerable daily intake (TDI) of 5 ng/kg BW/day (e.g. in small children who consume large quantities of milk).
According to Stoloff (1980), milk has the greatest demonstrated potential for introducing aflatoxin residues from edible animal tissues into the human diet. Research on AFM1 contamination of milk and milk products is copious and surely a greater body of information on this toxin exists than on any other (for extensive reviews see Fremy and Dragacci, 1999; Galvano et al., 1996).
In the last decade, the incidence of AFM1 contamination seems to have been reduced both by increasing the accuracy of detection procedures and through stricter regulatory limits (mainly in the EU) for AFB1 in feeds and AFM1 in milk. The incidence of AFM1 contamination is often higher in commercial milk than in raw farm milk, because of the pollution of uncontaminated bulk milk by a few contaminated samples. For the same reason, high AFM1 contamination levels in commercial milk seldom occurs. Nevertheless, the occurrence of AFM1 in cow’s milk and milk products is widespread, even if contamination levels do not seem to be a serious health hazard according to the current scientific fund of knowledge. The above considerations have been confirmed by recent surveys conducted in many countries (Carvajal et al., 2003a,b; Garrido et al., 2003; Roussi et al., 2002; Sivrastava et al., 2001; Oruc and Sonal, 2001; Galvano et al., 1998, 2001; Pietri et al., 2003). Since AFM1 may or may not be present in dairy products in a particular year depending on the weather conditions, wide and frequent monitoring programs using accurate and reliable analytical techniques remain the primary strategy to provide safe milk for consumers.
When dairy products are manufactured from milk contaminated with AFM1, the toxin is transmitted to the resulting products. AFM1 is stable in raw milk and processed milk products and is generally unaffected by pasteurization or processing into cheese, yogurt, cream and butter (Wood, 1991; Galvano et al., 1996). AFM1 combines with casein during the formation of cheese, resulting in higher concentrations of the toxin than are found in the whey. Concentrations exceed source milk levels by 2.5- to 3.3-fold in many soft cheeses and 3.9- to 5.8-fold in hard cheeses (Yousef and Marth, 1989).
Cheese can also be directly contaminated by toxins produced by undesired and/or intentionally added mycoflora that develop during ripening. However, the toxicological relevance of directly contaminated cheeses is still unknown.
Milk powder for infant formula is another route of exposure to AFM1. Galvano and co-workers (1996) reviewed the literature from 1980 to 1995. Although very little data are available in the scientific literature, authors concluded that the incidence and contamination level of AFM1 in dried milk and infant formulae should not have been a health hazard, and that, since infants are more vulnerable and sensitive than adults, monitoring of infant foods should be repeated more frequently and extensively. Since 1995 only six surveys have been conducted, two in Italy (Galvano et al., 1998; 2001) and one in Brazil, (Oliveira et al., 1997), Korea (Kim et al., 2000), Turkey (Aksit et al., 1997) and India (Rastogi et al., 2004). Consistent with data on liquid milk, overall low levels of contamination were reported with the exception of the study in India in which all samples exceeded the Commission of the European Community (EC) limit (50 ng/L). However, Aksit et al. (1997) correctly concluded that, although aflatoxin concentrations in the formulae were found to be within acceptable limits for most countries, its existence must be carefully evaluated, because the future effects of very small amounts of aflatoxin on growing organisms are unknown.
MEAT AND MEAT PRODUCTS
Meat and meat products from animals given mycotoxin contaminated feeds are a potential route of human exposure. However, meat from ruminant animals can be almost excluded as an important route of exposure for humans due to the degrading/converting action of rumen microflora that drastically reduces the carry-over to tissues. Among meats from non-ruminant animals pork is the most susceptible to mycotoxin contamination, specifically to OTA. Indeed, OTA can easily be transferred to pork due to its high incidence in pig feeds and the unfavorable elimination toxicokinetics that lead to a relatively long half-life in edible animal tissues.
OTA contamination of pork is a potential concern particularly in northern European countries and elsewhere where climatic conditions lead to a high incidence of OTA contamination in pig feeds. In Denmark, the implementation of post-mortem inspection programs has been an effective precautionary practice (Mousing et al., 1997); a level of 15 ppb of OTA in a pig liver or kidney results in its confiscation, and levels exceeding 25 ppb result in confiscation of the entire carcass. Surveys performed on pig meat in Denmark (Jorgensen and Petersen, 2002; Jorgensen, 1998) and Romania (Curtui et al., 2001), on sausages in Germany (Frank, 1991), on ham in Italy (Chiavaro et al., 2002), on pig liver in France (Dragacci et al., 1999) and on liver pates in Spain (Jimenez et al., 2001), reported the detection of acceptably low levels of OTA.
An evaluation of the sources of OTA intake in France demonstrated that the contribution of meat would be only ~3% of the total OTA intake (Verger et al., 1999).
Although pork meat is a route for human exposure to OTA and continuous surveillance is needed, its contribution is proportionately much less significant than that of some other foods.
EGGS
Mycotoxin residues in eggs have the potential to pose a significant human health hazard. Few studies are available on the carryover into eggs of mycotoxins other than AFB1, however residues of this toxin in eggs could potentially constitute an important human health hazard.
Aflatoxin B1 biotransformation in the liver of hens generates a variety of toxic hydroxylated metabolites that can also be transferred to eggs (Micco et al., 1987).
Widely variable (from 250:1 to 66200:1) transmission ratios (AFB1 in feed:AFB1 residual) for eggs have been reported in the literature (Galvano et al., 2005). In order to prevent high concentrations of AFB1 in eggs, since 1974, the EC has set a limit for AFB1 of 20 μg/kg for layer feed. A recent study (Pietri et al., 2001) indicated that if the official limit is respected, no trace of AFB1 or metabolites can be found in eggs. AFB1 residues detectable only in the low parts per trillion range can be found when the corresponding level in the feed is about 100 μg/kg. In examining these data and previously published information, it can be said that in Europe egg contribution to overall AFB1 intake is negligible.
Cereals for human use
Cereal grains and related by-products have a unique importance among food commodities because they are consumed by millions of people and are considered, from a nutritional point of view, the primary source of carbohydrates for humans, breeder animals, and farm livestock. Thus, microbiological, chemical, and mycotoxicological safety of cereals are considered very important for both the human and animal food chains.
Cereals are easily colonized by phytopathogenic moulds, which find excellent growth conditions in the field and during storage after harvest. The severe toxicities caused by moulds in animal and human tissues and organs are well known and cereals are a major route of introduction to the human food chain. Fungi belonging to the Fusarium, Penicillium, Alternaria and Aspergillus genera are involved in mycotoxin accumulation, whereas other genera such as Acremonium, Phomopsis, and Pithomyces are considered ancillary.
Important studies of fungi occurrence and mycotoxin production are available (e.g., D’Mello et al., 1997; Panigrahi, 1997; Smith, 1997). A great deal of attention has been given to aflatoxin producers such as A. flavus and A. parasiticus, because AFB1 is considered by the International Agency for Research on Cancer (IARC) to be the most carcinogenic compound produced by non-human activities. Consequently, many countries have legislated minimum levels for aflatoxins in foods, and worldwide regulations are in place for selected foods (Smith et al., 1997). The worldwide occurrence of aflatoxins in cereals is well documented, with the major contamination occurring in countries with high temperature and humidity. Corn is the most frequently contaminated cereal, whereas sorghum, rice, barley and wheat are less susceptible. Except for seasonal elevations in the contamination of corn, aflatoxin contamination of cereals rarely is a concern per se, although its frequent co-occurrence with combinations of other mycotoxins can cause serious problems. Recently Park and coworkers (2004) reported that rice is the major contributor to the dietary intake of AFB1 in Korea and calculated that the probable daily intake of AFB1 for Koreans exceeds the estimated provisional maximum tolerable daily intake.
Toxins produced by Fusarium fungi growing on cereals represent a serious concern, especially in temperate climates (Placinta et al., 1999). The most important and largest family of fusariotoxins are the trichothecenes, which consists of several compounds divided into four subgroups. Types A and B represent the most important members (D’Mello, 1997). Type A trichothecenes include T-2 toxin, HT-2 toxin, neosolaniol (NEO) and diacetoxyscirpenol (DAS), while type B trichothecenes include deoxynivalenol (DON or vomitoxin) and its 3-acetyl and 15-acetyl derivatives (3-ADON and 15-ADON, respectively), nivalenol (NIV) and fusarenon-X. The chemical structures of trichothecenes are related to Fusarium species, which permits chemotaxonomic identifications. Type A trichothecenes predominate in F. sporotrichioides and F. poae, whereas type B trichothecenes occur mainly in F. culmorum and F. graminearum. Cereal safety is directly related to trichothecene occurrence (Scott, 1989; Yoshizawa, 1991). Many outbreaks of acute human disease involving nausea, vomiting, gastrointestinal upset, dizziness, diarrhoea, and headache have been reported in Asia, which have been attributed to consumption of cereals contaminated with Fusarium species and, more recently, to the presence of DON at concentrations of 3,000 to 93,000 μg/kg of grain.
Occasionally, other trichothecenes were reported present as well, but at much lower incidence and concentration (WHO, 2002). As shown in Table 1, trichothecene occurrence in cereals, especially in corn, is a worldwide problem, both for highly industrialized countries such as Canada, Japan, the United States, Germany, and Norway and for developing countries such as South Africa, India, Vietnam.
Zearalenone is an important oestrogenic toxin that often co-contaminates cereals along with trichothecenes and moniliformin. Moniliformin is also synthesised by F. oxysporum, which in addition is a recognised source of the mycotoxins wortmannin and fusaric acid (D’Mello et al., 1997). The diversity of Fusarium mycotoxins is further illustrated by the production of fusarochromanones (TDP-1, TDP-2, and TDP-6) by F. equiseti, which synthesises several trichothecenes as well as zearalenone (Flannigan, 1991; D’Mello et al., 1997). Extensive data on global contamination of cereal grains indicate that Fusarium mycotoxins are not a secondary risk for consumer health (Muller and Schwadorf, 1993; Chulze et al., 1996; Viquez et al., 1996).
Since 1988, fumonisins, a novel mycotoxin family, have been the focus of surveillance by mycotoxicologists and chemists (Gelderblom et al., 1988; Marasas, 1995).
Fumonisins are mycotoxins produced by the fungus Fusarium moniliforme, which occurs naturally on corn. Fumonisins are structurally diverse compared with hundreds of other known mycotoxins; and in fact are very polar substances containing both amino and carboxylic groups. Table 2 provides surveillance data on fumonisin levels reported in selected countries.
VARIOUS CORN-BASED FOODS
Fumonisin B1 (FB1) is the most common fumonisin.
High health risk stems from corn simultaneously contaminated with FB1 and combinations of other mycotoxins, as reported in China (along with AFB1, Wang et al., 1995b), Vietnam (with DON, NIV and AFB1, Wang et al., 1995a), Indonesia (with AFB1, DON, NIV and ZEN, Ali et al., 1998), Korea (with DON, NIV, other trichothecenes and ZEN, Sohn et al., 1999; with aflatoxins and OTA, Park et al., 2002), Brazil (with aflatoxins and ZEN, Vargas et al., 2001; with aflatoxins, Ono et al., 2001;), Ghana (with aflatoxins, Kpodo et al., 2000), USA (with moniliformin, Gutema et al., 2000), Thailand (with aflatoxins, Yoshizawa et al., 1996) and southeast Asia (with aflatoxins, ZEN and nivalenol, Yamashita et al., 1995).
Recently, Cirillo and co-workers (2003a) reported frequencies and levels of contamination of DON and FB1 and FB2 in cereal-based foods in the Italian marketplace. In particular, of 202 samples investigated, including raw materials and processed cereal foods (bread, pasta, breakfast cereals, biscuits, baby and infant foods), 84% were contaminated with DON at levels between 7 and 930 μg/kg (median 65 μg/kg), 26% contained FB1 ranging from 10 to 2870 μg/kg (70 μg/ kg), and 35% contained FB2 at 10-790 μg/kg (80 μg/ kg). The highest levels of DON and FB1 were detected in raw cereals and wholemeal flours. The highest levels of FB2 were detected in durum wheat pasta. A widespread DON contamination was found in baby and infant foods at levels varying from 7 to 166 μg/kg.
Pietri and co-workers (2004), surveying corn grain produced in Italy over the period 1995 to 1999, found that FB1 was by far the most significant mycotoxin in this product. The average level was 3064 μg/kg; with 69.6% of samples containing over 1000 μg/kg, and 16.9% over 5000 μg/kg. Other studies have reported fumonisin occurrence in Italy, Portugal, and African countries (Doko et al., 1995). In Italy co-contamination of FB1 was observed with cyclodepsipeptides or sesterterpenes such as beauvericin or fusaproliferin (Ritieni et al., 1997).
Table 1. Selected data on worldwide trichothecenes and zearalenone contamination of cereals (μg/kg).
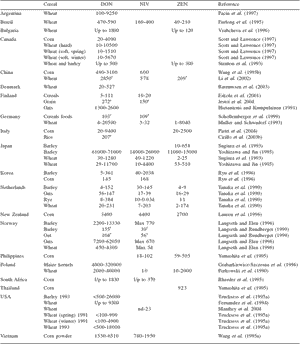
†Mean value
Table 2. Selected data on worldwide fumonisin contamination of corn (μg/kg).
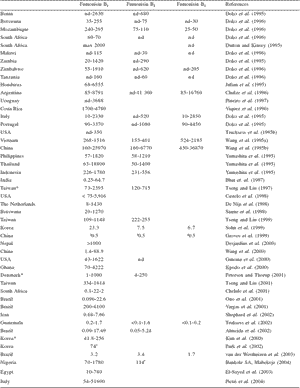
†Mean value. nd: not detectable
Wine and beer
The occurrence of OTA in wine was demonstrated for the first time by Zimmerli and Dick (1996) in a survey on 133 wines obtained from retail outlets in Switzerland.
The authors observed a higher OTA concentration in red wines than in white and rosé wines, thus deducing that the amount of OTA was dependent on the latitude of the production region: the further south the origin, the more frequent the occurrence and the greater the concentration. After the first detection of OTA in wine, several surveys were conducted, mainly in Europe (see Galvano et al., 2005 for extensive review). A clear geographic effect on OTA occurrence in red wines was demonstrated in Germany by Otteneder and Majerus (2000), in Italy by Pietri and co-workers (2001) and in Greece (Stefanaki et al., 2003). A general trend toward increasing OTA contamination for red wines from northern to southern latitudes was observed. Some of the above-mentioned authors noted that in general, lowcost red table wines had significantly higher OTA concentrations compared with good quality bottled wines. Generally, red wines are more contaminated than white wines due to differences in the wine-making process.
Obviously, OTA can contaminate not only wine, but also other grape products. Dried vine fruit (currants, raisins and sultanas) can be highly contaminated, thus can be an important dietary source of OTA for people (e.g., children) who have high levels of consumption.
Due to this, the Commission of the European Communities (2002) fixed an OTA limit of 10 μg/kg in dried vine fruits.
Unlike wine, which is almost exclusively contaminated by OTA, beer can be contaminated by a number of mycotoxins. Indeed, barley as well as other cereals (e.g. corn, wheat and sorghum) employed for producing beer, is a good substrate for the growth of many toxigenic fungi. Consequently, beer can be contaminated by all of the major mycotoxins except perhaps by citrinin, which does not seem to survive the mashing step. Data on the occurrence of mycotoxins in beer were recently reviewed by Galvano and co-workers (2005). The authors concluded that, for many reasons, beer contamination is worldwide, however due to differences in analytical procedures it is very difficult to compare the data. Nevertheless, with few exceptions, overall reported contamination levels are low.
A number of factors are responsible for the great variability in the types and amounts of mycotoxin contaminants in beer. Climatic conditions and cereal sources are the most decisive factors that can selectively favor the growth of a particular mould. These factors also explain the clear geographical patterns. The importance of other factors, such as storage and pretreatment of cereal source and brewery procedures, are yet to be fully understood. In addition, the possible co-occurrence of mycotoxins in beer is an issue that remains to be investigated. In some geographic areas, beer is a significant dietary component and must be considered in estimating the probable daily intake of mycotoxins other than OTA.
Fruits and fruit juices
Fruits and vegetables are another important part of a balanced human diet, but they may be colonised by moulds that produce mycotoxins (Drusch and Ragab, 2003). A common mycotoxin in apple juice and to a lesser extent in grape juice is citrinin, produced by Penicillium expansum (Vinas et al., 1993). In addition, OTA biosynthesized by Aspergillus carbonarius is a significant risk for apple and grape juice (Pitt, 2000).
Patulin is considered the most dangerous mycotoxin in fruit juices, and is produced by several fungal species such as B. fulva, B. nivea and P. expansum, which are the major patulin-producing microorganisms (Frisvad and Thrane, 1996). Patulin is partially destroyed by the fermentation process during cider production (Ough and Corison, 1980; Stinson et al., 1978). Patulin has been widely studied in fruit juices (Mortimer et al., 1985; Larsen et al., 1998; de Sylos and Rodriguez-Amaya, 1999), particularly in apple juice (Scott et al., 1972; Mortimer et al., 1985; de Sylos and Rodriguez-Amaya, 1999; Beretta et al., 2000). More recently, patulin and chaetoglobosin surveys were conducted by Ritieni (2003) and Andersen and co-workers (2004). Patulin occurrence in fruit juices is related to several factors such as temperature and water activity (Northolt et al., 1996) or technological treatments during transformation (Jackson et al., 2003). In addition, agronomic practices in fruit cultivation and juice making have a great influence on the occurrence of both patulin and citrinin (e.g., in Portuguese apples; Martins et al., 2002). Many research groups have recommended that apple products for human consumption should not contain residual patulin levels in excess of 50 μg/L.
Bananas can be contaminated by mycotoxins produced by the Fusarium species. In fact, bananas can be invaded after harvest by various fungal species that produce ‘crown rot’ (Ogundero 1987). F. moniliforme isolates have been found to produce potentially three 12,13- epoxytrichothecene mycotoxins (trichothecolone, diacetoxyscirpenol (DAS), and T-2 toxin), the palmitoyl esters of trichothecolone, scirpenetriol, and T-2 tetraol (T-2 TOL), and free and palmitic acid-conjugated zearalenone (Chakrabarti et al., 1986). Finally, Shenasi and co-workers examined 25 varieties of dates (Phoenix dactylifera) at different maturation stages for aflatoxins and aflatoxigenic Aspergillus spp. The samples were examined as fresh fruit and under simulated storage conditions of high humidity. Aflatoxins were detected in 12% of the samples and aflatoxigenic Aspergilli were detected in 40% of the varieties examined (Shenasi et al., 2002).
Coffee and cocoa products
Coffee can contain OTA produced by Aspergillus ochraceus or A. carbonarius. Studies in Brazil have shown that mould growth and OTA production occur only during drying of green coffee beans, and that if drying is rapid and effective, OTA will not be produced.
OTA has been found in green coffee for several years, but there has been and still is some uncertainty as to what extent OTA is degraded during the roasting process and further transmitted from roasted coffee to the final coffee brew. The reported reductions caused by roasting vary, but the major source of variation was a low OTA level (generally less than 10 μg/kg) in green coffee submitted to the process. Reports usually showed high rates of OTA reduction, ranging between 30 and 90%. Decaffeination seems to be an effective process, resulting in 92% reduction of OTA. During freeze-dried coffee manufacture or brewing, nearly 80% of OTA initially present in roasted and ground coffee was found to be transferred to the cup (van der Stegen et al., 2001).
Many surveys of roasted coffee demonstrated that OTA incidence is generally low and that levels exceeding 5 μg/kg are very rare. Recent data obtained in Germany, the UK, and Switzerland seem to indicate that coffee on the European market is contaminated with a mean OTA level of around 0.8 μg OTA/kg. On the basis of available contamination rates and mean consumption of food categories, it was calculated that coffee contributed an average of 2 to 3 ng/kg, while cereals and wine contributed about 25 and 10 ng/kg BW per week, respectively (WHO, 2001).
Cocoa beans are the source of cocoa powder, which is a frequent ingredient in several kinds of foods, cakes, biscuits, children’s foods, ice creams, and sweets. The agronomic and storage conditions associated with cocoa beans are favourable for mould growth, mainly Penicillium spp., and consequently OTA biosynthesis is not unexpected. An initial study on OTA in cocoa found that it was present at detectable levels in 67% of analysed samples. There was no correlation between OTA and visible mould on the beans, or geographic source of the beans. OTA was found at lower levels in cocoa butter than in the non-fat fraction (powder or cake) (Beckett, 1994). In 1998, a survey was conducted to assess the exposure of UK consumers to OTA in cocoa powder and chocolate (http://www.britanniafood.com). The OTA contamination in chocolate bars and cocoa powder was generally low, although OTA was detected in all 20 samples of cocoa powder analysed and three out of four chocolate bars. Considering chocolate samples, 75% of samples were positive for OTA, but at low levels. It was concluded that there is little cause of concern about the level of OTA in cocoa and chocolate.
Spices
Spices are other foods that have the potential to be highly contaminated, especially by AFB1. However, their toxicological importance is generally considered to be low relative to their quantitative inclusion in the diet.
Suspected human mycotoxicosis
Diseases caused by mycotoxins are called mycotoxicoses and can be acute, chronic, or subchronic. Acute cases of mycotoxicosis are rare and limited to developing countries. Conversely, long-term exposure to low levels of mycotoxins is an insidious, widespread problem that scientists are called to address worldwide. Indeed, chronic mycotoxicoses are difficult to recognize, as epidemiological evidence is difficult to demonstrate due to the involvement of multiple epidemiological factors and uncertainties in assessing exposure. Although sufficient evidence of the involvement of mycotoxins as etiological agents of human diseases arises from both laboratory and epidemiological studies, caution is needed when blaming mycotoxins for human disease because the literature is inconclusive in establishing direct and unequivocal causal relationships.
The first historically recorded case of human mycotoxicosis was ergotism, also known as St. Anthony’s Fire, caused by the ingestion of grains contaminated by sclerotia of Claviceps purpurea. After periodic outbreaks, the disease became epidemic in central Europe during the Middle Ages. Nowadays risk of ergotism seems to be limited to animals. The last reported cases in humans occurred in the 1970s.
Tricothecenes are suspected to be responsible for two diseases: alimentary toxic aleukia and Kashin Beck disease; and citreoviridin ingestion has been linked with cardiac beriberi. Two recent epidemiological suspicions of mycotoxin involvement in a human disease have also been reported. The first involves OTA in the etiology of testicular cancer, as hypothesized by Schwartz (2002).
The second involves fumonisins as a causative agent in human neural tube defects. Marasas and co-workers (2004) proposed that fumonisins affect folate utilisation thus making them potential risk factors for birth defects arising from neural crest cells.
Sufficient epidemiological data support the theory that three mycotoxins are strongly suspected or directly involved in the etiology of specific human diseases with high regional incidence. These are OTA associated with human nephropathies, aflatoxin B1 for hepatocarcinoma, and fumonisins for esophageal cancer, as summarized below.
OCHRATOXIN AND NEPHROPATHY
OTA is suspected to be involved in the etiology of human nephropathies and tumors of urinary organs. The persistence of OTA in the human body is prolonged as it has a blood half-life of 35 days after a single oral dosage, due to unfavorable elimination toxicokinetics (Studer-Rohr et al., 2000). The long half-life of OTA, together with frequent exposure of humans by ingestion of OTA-contaminated food, results in a high frequency of OTA in human blood samples collected around the world (Speijers and van Egmond, 1993; WHO, 2001).
OTA is neither stored nor deposited in the body, but numerous laboratory and animal studies clearly demonstrate that it is distributed via the blood mainly to the kidneys (Hult and Fuchs, 1986). In several mammalian species, even at low doses, OTA is nephrotoxic, which is likely to hold true in humans.
Despite the numerous epidemiological studies performed, the causality of OTA exposure with human nephropathies has never been proven (WHO, 2001).
The usual investigative approach is to analyse OTA in foods and in biological fluids (mainly plasma) of a healthy population and/or restricted groups of patients suffering from nephropathies. Many studies conclude a possible involvement of OTA in the etiology of a fatal human disease referred to as Balkan Endemic Nephropathy (BEN), which is found in some regions of Bosnia and Herzegovina, Bulgaria, Croatia, Serbia, and Romania (Petkova-Bocharova et al., 2002). First in Bulgaria, and afterwards in other countries where BEN is present, an unusually high incidence of urinary tract tumors was noted (Ceovic et al., 1992; Pfohl- Leszkowicz et al., 2002). Several epidemiological studies have been conducted in countries in northern Africa, such as Egypt (Wafa et al., 1998), Algeria (Khalef et al., 1993), Morocco (Filali et al., 2002) and, particularly, Tunisia (Eko-Ebongue, 1994; Maarou et al., 1995a,b) to establish a causal relationship between OTA and a human nephropathy similar to BEN, and tentatively called chronic interstitial nephropathy (CIN).
Two contradictory studies from Tunisian researchers (Abid et al., 2003; Grosso et al., 2003) have been published, showing that causal uncertainty still exists.
Data collected by Abid et al. (2003) over the period 1991–2000 on a quite large number of volunteers, supported OTA involvement in the Tunisian CIN. Additional supporting evidence came from the detection of significant levels of OTA in plasma of human groups living in conditions similar to those in the Balkans and consuming OTA-contaminated foods. However Grosso and co-workers (2003) came to the opposite conclusion by observing OTA levels in sera from volunteers hospitalized for kidney damage that were not very high.
Recently, Hassen and co-workers (2004) found that high blood OTA and β(2)-microglobulinuria levels seem strongly associated with CIN, thus supporting OTA involvement in the outcome of CIN and underlining the importance of β(2)-microglobulinuria in the characterisation of this disease. According to Abouzied and co-workers (2002), OTA alone could not induce BEN, but it could act synergistically with other environmental toxins. A new approach, based on the duplicate-diet method, was reported by Vrabcheva and co-workers (2004). In the attempt to confirm the exposure of a population to OTA in the Vratza district in Bulgaria, these researchers followed the frequency of consumption of OTA-contaminated food by selected families in the BEN area and the OTA intake level over a one-month period. Results correlated the extremely high OTA exposure of the population from this BEN area, and implicated its involvement in this disease.
Despite intensive research at both epidemiological and experimental/mechanistic levels, it is still an open question as to whether OTA plays a causative or subordinate role in the induction of human nephropathies worldwide. Differences related to: 1) detection limit and overall analytical reliability of OTA measurement in biological fluids, and 2) qualitative (e.g., kind and severity of nephropathy) and quantitative sampling of the population as well as of foods, likely make results not easily comparable. Seasonal variations of OTA in foods and consequently in sera should be also considered, as demonstrated by Peraica and co-workers (2001) in a survey of a Croatian population. Finally, but equally important, it will be necessary to test possible synergistic effects of mycotoxins (e.g., citrinin) simultaneously present with OTA in foods. For example, in a study by Vrabcheva and co-workers (2000) levels of citrinin were detected up to 200-fold higher than OTA in cereals from Bulgarian villages with a history of Balkan endemic nephropathy.
AFLATOXIN AND HEPATOCELLULAR CARCINOMA
Hepatocellular carcinoma (HCC) is one of the most widespread malignancies. It has the fourth highest mortality rate worldwide and is estimated to cause approximately half a million deaths annually. Geographic areas of interest include Asia, southern China, and sub- Saharan Africa. An increasing incidence of HCC has also recently been observed in the United States (el Serag and Mason, 1999). Chronic hepatitis B or C infections, exposure to dietary AFB1 and alcoholic cirrhosis have been demonstrated to be, in order of importance, the major risk factors in the multifactorial etiology of HCC.
Early epidemiological studies conducted in areas with high HCC incidence basically investigated the possible correlation between AFB1 dietary exposure and the occurrence of HCC. The involvement of other factors, such as hepatitis B virus (HBV) infection, was verified only in successive studies. Recently, the possibility of a synergistic carcinogenic interaction between HBV chronic infection and dietary exposure to AFB1 arose from the observation of their co-existence in countries with high incidence of HCC and was confirmed by further experimental and epidemiological studies (Kew, 2003). Sufficient data led to the conclusion that chronic hepatitis B or C infections, exposure to dietary AFB1, and alcoholic cirrhosis are, in order of importance, the major risk factors in the multifactorial etiology of HCC.
However, the carcinogenic potency of AFB1 is considered much lower in populations where chronic hepatitis infections are rare. Thus, recommendations for the control of aflatoxicosis have been proposed.
FUMONISIN AND OESOPHAGEAL CANCER
In areas where humans consume corn as the staple food, fumonisin contamination has been linked with oesophageal cancer. The usual methodological approach consists of verifying the relationship between fumonisincontaminated corn and the incidence of oesophageal cancer, often by comparing data from high and low risk areas. The countries where this relationship has been more thoroughly investigated are South Africa (especially the Transkei region) (Chelule et al., 2001; Marasas, 2001) and China (Qiu et al., 2001; Abnet et al., 2001; Chu and Li, 1994; Wang et al., 2000; Yoshizawa et al., 1994). However, the possible role of fumonisin-contaminated corn has been also investigated in other areas with high oesophageal cancer incidence such as northeastern Italy (Franceschi et al., 1995; Visconti and Doko, 1994), southern Brazil (van der Westhuizen et al., 2003), and Iran (Shephard et al., 2002). In a more recent approach investigators tried to establish a relationship between the altered ratio of sphingolipids (e.g., sphinganine:sphingosine, Sa:So ratio) and oesophageal cancer incidence. Indeed, fumonisin B1, structurally resembling sphingoid bases, is an inhibitor of ceramide synthetase, a key enzyme involved in de novo sphingolipid synthesis and in the reacylation of free sphingoid bases derived from sphingolipid turnover. This inhibitory effect leads to accumulation of free sphinganine and sphingosine and alteration of the Sa:So ratio. This ratio has been demonstrated to be an early biomarker of fumonisin exposure that is relatively easy to analyse in the urine.
Based on this finding, Qiu and co-workers (2001) found that the urinary Sa:So ratio in men in the Chinese endemic area of Henan province was significantly higher than that of the population in Suixian, a low oesophageal cancer incidence area, whereas no difference was observed in women. Diversely, Abnet and co-workers (2001) found no significant association between serum sphingolipid levels and risk of oesophageal cancer in Linxian (China).
In 1993, the IARC concluded there is ‘inadequate evidence’ for carcinogenicity in humans from oral exposure to FB1 and a role for fumonisins in any other human disease has likewise not been proven. However, the accumulated data over the last decade demonstrate that known naturally occurring levels of fumonisin present a potential threat to human health and also suggest that fumonisin can cause oesophageal cancer.
As underlined in the conclusions of the recent task force report of the US Council for Agricultural Science and Technology (CAST, 2003), additional research into the relationships between fumonisin, sphingolipid metabolism disruption, and apoptosis is necessary for understanding the potential carcinogenicity of fumonisin, and determining the relevance of these mycotoxins to human health.
Human exposure and risk assessment
The danger of mycotoxins and their pervasiveness have led public authorities to adopt strict control policies. The overall scientific approach consists of coupling exposure data of an individual or population with toxicological data. In the estimation of exposure levels the occurrence of a mycotoxin in a food and its dietary burden are taken in account. Toxicological data are obtained from laboratory studies on animals and safety/ uncertainty factors (multiples of 10) are used to extrapolate data on laboratory animals to humans and to provide an acceptable or tolerable daily intake (ADI/ TDI).
On the basis of the available toxicological data and using the five Global Environmental Monitoring System/ Food Contamination Monitoring and Assessment Programme (GEMS/Food) regional diets (WHO, 1998), the Joint Expert Committee on Food Additives (JEFCA) of the World Health Organization and of the Food and Agriculture Organization, has recently evaluated the hazard for the most significant mycotoxins (e.g., OTA, fumonisins, DON, T-2 toxin, patulin, and zearalenone) and established the Provisional Maximum Tolerable Weekly Intake (PMTWI) and the Provisional Maximum Tolerable Daily Intake (PMTDI) (JEFCA, 2001). These values are obtained by calculations subsequent to the quantification, by animal studies, of the No Observed Adverse Effect Level (NOAEL). The NOAEL is then divided for a safety/uncertainty factor of 100 (10 for extrapolation of animal data to humans and 10 for individual variation). As clearly indicated by the term ‘provisional’, the values cannot be considered definitive.
In view of the further quantification of TDI, it is necessary to establish Risk Assessment, which is the product of hazard assessment and exposure assessment. The procedure includes the following steps: 1) hazard assessment based on animal toxicity and possibly epidemiological data, 2) exposure assessment based on data concerning actual concentrations in commodities, 3) extrapolation of hazard assessment from highexposure animal data to low-exposure human data, and 4) comparison of the product of hazard assessment and exposure assessment with acceptable risk (Kuiper- Goodman, 1995). In the estimation of probable human exposure to a mycotoxin, all potentially contaminated food sources must be considered. The estimated PMTWI or PMTDI and the exposure data allow a maximum permissible limit for a specific commodity or range of foodstuffs to be established. However, due to the great worldwide variability in dietary patterns, it is difficult to harmonize limits. Indeed, a highly contaminated staple food consumed in one region requires a limit that would be too severe for the same food in an area where dietary intake of that food is low.
AFLATOXIN
A different risk assessment approach was used for genotoxic mycotoxins such as AFB1. As AFB1 has carcinogenic properties, and assuming that a no-effect concentration limit cannot be established for genotoxic compounds, any dose will have a proportional effect.
Thus, JECFA did not establish a PMTWI or PMTDI, but recommended that its level in food should be As Low As Reasonably Achievable (ALARA). In regard to AFM1, there is sufficient evidence that AFM1 is a genotoxic carcinogen, although its carcinogenic potency is estimated to be approximately 10 times lower than AFB1. Nevertheless, as milk and milk products are heavily consumed by humans, particularly by infants and young children, the risks from aflatoxin exposure need careful consideration. Thus, JEFCA assessed an estimation of dietary AFM1 intake. Highest contamination levels were found in samples from the Far Eastern region, where milk consumption is generally low. The Committee concluded that even with the worstcase assumptions, the additional risks for liver cancer predicted with use of the proposed maximum levels of AFM1 are very small, even for big milk drinkers.
OCHRATOXIN A
Data on mycotoxin exposure and/or risk assessment are available in the literature for OTA and different tolerable daily intakes (TDI) have been suggested over the last decade. In 1998, the European Commission’s Scientific Committee on Food (SCFOO, 1998) recommended that it would be prudent to minimize exposure to OTA as much as possible, e.g., to below 5 ng/kg/BW/day. A cautious TDI (5 ng/kg bw) has been also proposed by the Working Group of the Nordic Council of Ministers (Olsen et al., 1991), whereas the Canadian authority proposed a TDI in the range 1.2 to 5.7 ng/kg BW (Kuiper-Goodman, 1996). These differences in recommendations also reflect differences in risk management measures, resulting in varying legal limits applied to different commodities and to the same commodity in different countries (Walker, 2002). In any case, as pointed out by Skaug (1999), to date OTA risk assessments do not differentiate between risk to adults and children. The latter represent a particularly sensitive population that warrants a customized TDI, considering the unfavorable dose/body weight ratio.
FUMONISINS
In 2003, JEFCA established a group TDI of 2 μg/kg BW for the total of fumonisin B1, B2, and B3, alone or in combination. However, using GEMS/Food regional diets data, the fumonisin intake of people in the African region is 2.4 μg/kg BW/day based on mean levels of corn contamination. According to South African researchers at the PROMEC unit, the TDI established by JECFA is appropriate to protect consumers in the developed countries, where corn consumption is low, whereas much lower tolerance levels of 200 μg/kg would be required to protect subsistence farming communities that consume corn as a staple food in developing regions, such as Transkei (Marasas, 1997; Shephard et al., 2002). Additional uncertainties in establishing risk assessment for fumonisin depend on the great seasonal and geographic fluctuation of contamination levels. For example, when JECFA used estimates of contamination at the higher end of the scale (90th percentile), four out of five regions (Middle East, the Far East, Latin America and Africa) exceed the PMTDI, with Africa reaching an alarming 7.3 mg/kg BW per day. Experts from the Nordic countries (Denmark, Norway, Sweden, Finland, and Iceland) have conducted a preliminary evaluation of fumonisins concluding that, at that time, it was not possible to conduct a complete risk assessment. However, they recommended that the human daily intake of fumonisins should be less than 1 μg/kg BW/day (Petersen and Thorup, 2001).
DON AND FUSARIOTOXINS
The joint FAO/WHO Expert Committee on Food Additives (JECFA, 2001) established for DON a PMTDI of 1 μg/kg BW and a group PMTDI of 0.06 μg/kg BW for T-2 toxin and HT-2 toxin. However, data were only available for the European region, so that more accurate information from other parts of the world and improved analytical methods are needed. In a study conducted in the Netherlands, Pieters and co-workers (2002) derived a provisional TDI of 1.1 μg/kg BW and proposed a concentration limit of 129 μg DON/kg wheat based on this TDI and the high wheat consumption of children. The authors highlighted that in the period September 1998 through January 2000, due to high DON concentration in wheat, the dietary intake of DON exceeded the provisional TDI, especially in children, and that negative health effects could occur. In France, Leblanc and co-workers (2002) showed that consumers of high amounts of organically produced cereals can exceed the PTDI for DON over their lifetime. Very recently Schothorst and Van Egmond (2004) presented the results of the SCOOP (Scientific Co-operation on Questions relating to Food) subcommittee on trichothecenes. Despite a significant lack of consumption data in some countries and very poor information on infant and children’s foods, the authors concluded the following: 1) although among cereals, corn showed the highest level of contamination with trichothecenes, wheat and wheat-containing products (like bread and pasta) represent the major source of intake; 2) the overall mean intake for DON is below the TDI, although for young children the mean intakes are sometimes (very) close to the TDI; 3) NIV intakes are far below the TDI; and 4) T-2 and HT-2 toxin intakes are in most cases above the TDI.
Conclusions
Mycotoxins in the human food chain are an unavoidable problem that encompasses, by varying degrees, virtually all foods and all people. A basic distinction must be drawn between developing and developed countries. In the first, people are surely exposed to both acute and chronic mycotoxicosis. However, at least in the poorest developing countries where the fundamental problem is to provide a sufficient food supply, it appears unrealistic to address food safety problems. People living in developed countries are surely less exposed to mycotoxins than those in developing countries. This is due to several factors, e.g. abundance of food resources, modern food handling and preservation technology, and effective regulation and control of food quality.
However, commodities imported from developing countries can increase the overall risk of exposure if strict regulatory policies are not enforced. In developed countries, apart from peculiar regional circumstances, the overall situation is characterised by widespread contamination at low levels, leading to human health risk related to chronic exposure.
Except for AFM1, which is within tolerable limits of consumption, available data show that in a considerable part of the world PMDTIs are exceeded for fumonisin and DON. It is also reasonable to hypothesize that further acquisition of data could show that dangerous levels of T-2/HT-2 and OTA are being ingested. Additional warnings arise from the fact that the single mycotoxin approach in the definition of PMDTI ignores the potentially additive and/or synergic toxicity of diverse mycotoxins that very frequently coexist in foods, especially cereals. Considering several factors such as:
1) frequency and levels of contamination;2) relative importance of the food in a diet; and 3) available toxicological information, some peculiar situations are worthy of consideration. OTA seems to be, among the most common mycotoxins, the one that should be monitored with the maximum attention because of its occurrence in almost all foods, thus leading to potentially high total dietary intake. In a hypothetical contaminated foods warning list, cereals should be listed first, because of their worldwide dietary importance, and because of the demonstrated natural co-occurrence of almost all the possible combinations of the major mycotoxins, at sometimes elevated levels. As a final concern among human categories, children merit the utmost consideration by researchers and public authorities because their diets lack diversity, their unfavourable exposure dose:body weight ratio, and their greater sensitivity.
Researchers are called to gather more toxicological and epidemiological information. The detection of biomarkers in biological fluids can be an useful tool for establishing effective mycotoxin exposure levels, as confirmed by the growing interest of scientists in this field. Dietary strategies that can prevent or reduce the toxic effects of mycotoxins should also be further explored. Public authorities are charged to set worldwide harmonious regulatory limits, avoiding economic special interests.
References
Abdulrazzaq, Y.M., N. Osman, Z.M. Yousif and S. Al- Falahi. 2003. Aflatoxin M1 in breast-milk of UAE women. Ann. Trop. Paediatr. 23:173-179.
Abid, S., W. Hassen, A. Achour, H. Skhiri, K. Maaroufi, F. Ellouz, E. Creppy and H. Bacha. 2003. Ochratoxin A and human chronic nephropathy in Tunisia: is the situation endemic? Hum. Exp. Toxicol. 22:77-84.
Abnet, C.C., C.B. Borkowf, Y.L. Qiao, P.S. Albert, E. Wang, A.H. Merrill, Jr., S.D. Mark, Z.W. Dong, P.R. Taylor and S.M. Dawsey. 2001. Sphingolipids as biomarkers of fumonisin exposure and risk of esophageal squamous cell carcinoma in China. Cancer Causes Control 12:821-828.
Abouzied, M.M., A.D. Horvath, P.M. Podlesny, N.P. Regina, V.D. Metodiev, R.M. Kamenova-Tozeva, N.D. Niagolova, A.D. Stein, E.A. Petropoulos and V.S. Ganev. 2002. Ochratoxin A concentrations in food and feed from a region with Balkan Endemic Nephropathy. Food Addit. Contam. 19:755-764.
Aksit, S., S. Caglayan, I. Yaprak and S. Kansoy. 1997. Aflatoxin: is it a neglected threat for formula-fed infants? Acta Paediatr. Jpn. 39:34-36.
Ali, N., Sardjono, A. Yamashita and T. Yoshizawa. 1998. Natural co-occurrence of aflatoxins and Fusarium mycotoxins (fumonisins, deoxynivalenol, nivalenol and zearalenone) in corn from Indonesia. Food Addit. Contam. 15:377-384.
Almeida, A.P., H. Fonseca, A.L. Fancelli, G.M. Direito, E.M. Ortega and B. Correa. 2002. Mycoflora and fumonisin contamination in Brazilian corn from sowing to harvest. J. Agric. Food. Chem. 50:3877- 3882.
Andersen, B., J. Smedsgaard and J.C. Frisvad. 2004. Penicillium expansum: consistent production of patulin, chaetoglobosins, and other secondary metabolites in culture and their natural occurrence in fruit products. J. Agric. Food Chem. 21:52(8):2421- 2428.
Bankole, S.A. and O.O. Mabekoje. 2004. Occurrence of aflatoxins and fumonisins in preharvest maize from southwestern Nigeria. Food Addit. Contam. 21:251- 255.
Beckett, S.T. 1994. Industrial Chocolate Manufacture and Use (2nd ed). Blackie Academic and Professional, Chapman and Hall, London. Beretta, B., A. Gaiaschi, C.L. Galli and P. Restani. 2000. Patulin in apple-based foods: Occurrence and safety evaluation. Food Addit. Contam. 17(5):399-406.
Bhat, R.V., P.H. Shetty, R.P. Amruth and R.V. Sudershan. 1997. A foodborne disease outbreak due to the consumption of moldy sorghum and maize containing fumonisin mycotoxins. J. Toxicol. Clin. Toxicol. 35:249-255.
Breitholtz-Emanuelsson, A., M. Olsen, A. Oskarsson, I. Palminger and K. Hult. 1993. Ochratoxin A in cow’s milk and in human milk with corresponding human blood samples. J. AOAC Int. 76:842-846.
Carvajal, M., A. Bolanos, F. Rojo and I. Mendez. 2003a. Aflatoxin M1 in pasteurized and ultrapasteurized milk with different fat content in Mexico. J. Food Prot. 66:1885-1892.
Carvajal, M., F. Rojo, I. Mendez and A. Bolanos. 2003b. Aflatoxin B1 and its interconverting metabolite aflatoxicol in milk: the situation in Mexico. Food Addit. Contam. 20:1077-1086.
Council for Agricultural Science and Technology. 2003. Mycotoxins: Risks in Plant, Animal, and Human Systems. Task Force Report, ISSN 0194-4088, Ames, Iowa, USA.
Castelo, M.M., S.S. Sumner and L.B. Bullerman. 1998. Occurrence of fumonisins in corn-based food products. J. Food Prot. 61:704-707.
Ceovic, S., A. Hrabar and M. Šaric. 1992. Epidemiology of Balkan endemic nephropathy. Food Chem. Toxicol. 30:183-188. Chakrabarti, D.K. and S. Ghosal. 1986. Occurrence of free and conjugated 12,13-epoxytrichothecenes and zearalenone in banana fruits infected with Fusarium moniliforme. Appl. Environ. Microbiol. 51:217–219.
Chelule, P.K., N. Gqaleni, M.F. Dutton and A.A. Chuturgoon. 2001. Exposure of rural and urban populations in KwaZulu Natal, South Africa, to fumonisin B1 in maize. Environ. Health Perspect. 109:253-256.
Chiavaro, E., A. Lepiani, F. Colla, P. Bettoni, E. Pari and E. Spotti. 2002. Ochratoxin A determination in ham by immunoaffinity clean-up and a quick fluorometric method. Food Addit. Contam. 19:575- 581.
Chu, F.S. and G.Y. Li. 1994. Simultaneous occurrence of fumonisin B1 and other mycotoxins in moldy corn collected from the People’s Republic of China in regions with high incidences of esophageal cancer. Appl. Environ. Microbiol. 60:847-852.
Chulze, S.N., M.L. Ramirez, M.C. Farnochi, M. Pascale, A. Visconti and G. March. 1996. Fusarium and fumonisin occurrence in Argentinean corn at different ear maturity stages. J. Agric. Food Chem. 44:2797-2801.
Cirillo, T., A. Ritieni, F. Galvano and R. Amodio Cocchieri. 2003a. Natural co-occurrence of deoxynivalenol and fumonisins B1 and B2 in Italian marketed foodstuffs. Food Addit. Contam. 20:566- 571.
Cirillo, T., A. Ritieni, M. Visone and R.A. Cocchieri. 2003b. Evaluation of conventional and organic Italian foodstuffs for deoxynivalenol and fumonisins B(1) and B(2). J Agric. Food Chem. 51:8128-8131.
Commission of the European Communities. 2002. EC Regulation 2002/472, 12.03.2002, Official Journal of the EC, L75/18.
Curtui, V.G., M. Gareis, E. Usleber and E. Martlbauer. 2001. Survey of Romanian slaughtered pigs for the occurrence of mycotoxins ochratoxins A and B, and zearalenone. Food Addit. Contam. 18:730-738.
de Nijs, M., E.A. Sizoo, A.E. Vermunt, S.H. Notermans and H.P. van Egmond. 1998. The occurrence of fumonisin B1 in maize-containing foods in The Netherlands. Food Addit. Contam. 15:385-388.
de Sylos, C.M. and D.B. Rodriguez-Amaya. 1999. Incidence of patulin in fruits and fruit juices marketed in Campaninas, Brazil. Food Addit. Contam. 16(2):71-74.
Desjardins, A.E., G. Manandhar, R.D. Plattner, C.M. Maragos, K. Shrestha and S.P. McCormick. 2000. Occurrence of Fusarium species and mycotoxins in Nepalese maize and wheat and the effect of traditional processing methods on mycotoxin levels. J. Agric. Food Chem. 48:1377-1383.
D’Mello, J.P.F., J.K. Porter, A.M.C. Macdonald and C.M. Placinta. 1997. Fusarium mycotoxins. In: Handbook of Plant and Fungal Toxicants (J.P.F. D’Mello, ed). CRC Press, Boca Raton, FL, USA, pp. 287-301.
Doko, M.B., C. Canet, N. Brown, E.W. Sydenham, S. Mpuchane and B.A. Siame. 1996. Natural cooccurrence of fumonisins and zearalenone in cereals and cereal-based foods from eastern and southern Africa. J. Agric. Food Chem. 44:3240-3243.
Doko, M.B., S. Rapior, A. Visconti and J.E. Schjoth. 1995. Incidence and levels of fumonisin contamination in maize genotypes grown in Europe and Africa. J. Agric. Food Chem. 43:429-434.
Dragacci, S., F. Grosso, R. Bire, J.M. Fremy and S.A. Coulon. 1999. French monitoring programme for determining ochratoxin A occurrence in pig kidneys. Nat. Toxins 7:167-173.
Drusch, S. and W. Ragab. 2003. Mycotoxins in fruits, fruit juices, and dried fruits J. Food Prot. 66:1514- 1527.
Dutton, M.F. and A. Kinsey. 1995. Occurrence of mycotoxins in cereals and animal feedstuffs in Natal, South Africa 1994. Mycopathologia 131:31-36.
Eko-Ebongue, S., J.P. Antalick, M. Bonini, A.M. Betbeder, J.G. Faugere, K. Maarou, H. Bacha, M. Achour, Y. Grosse, A. Pfohl-Leszkowicz and E.E. Creppy. 1994. Determination des teneurs en ochratoxine A, vitamine E et vitamine A dans les sérums humains de France et de Tunisie: recherche d’une correlation. Annales de Falsification de l’Expertise Chimique 929:213-224.
el-Nezami, H.S., G. Nicoletti, G.E. Neal, D.C. Donohue and J.T. Ahokas. 1995. Aflatoxin M1 in human breast milk samples from Victoria, Australia and Thailand. Food Chem. Toxicol. 33:173-179.
el-Sayed, A.M., E.A. Soher and A.F. Sahab. 2003. Occurrence of certain mycotoxins in corn and cornbased products and thermostability of fumonisin B1 during processing. Nahrung. 47:222-225.
el-Serag, H.B. and A.C. Mason. 1999. Rising incidence of hepatocellular carcinoma in the United States. N. Eng. J. Med. 340:745-750.
Eskola, M., P. Parikka and A. Rizzo. 2001. Trichothecenes, ochratoxin A and zearalenone contamination and fusarium infection in Finnish cereal samples in 1998. Food Addit. Contam. 18:707-718.
Fernandez, C., M.E. Stack and S.M. Musser. 1994. Determination of deoxynivalenol in 1991 U.S. winter and spring wheat by high-performance thin-layer chromatography. J. AOAC Int. 77:628-630.
Filali, A., A.M. Betbeder, I. Baudrimont, A. Benayad, R. Soulaymani and E.E. Creppy. 2002. Ochratoxin A in human plasma in Morocco: a preliminary survey. Human Exper. Toxicol. 21:241-245.
Flannigan, B. 1991. Mycotoxins. In: Toxic Substances in Crop Plants (J.P.F. D’Mello, C.M. Duffus and J.H. Duffus, eds). The Royal Society of Chemistry, Cambridge, pp. 226-257.
Franceschi, S., E. Bidoli, A.E. Barón and C. La Vacchia. 1995. Maize and risk of cancer of the oral cavity, pharynx and esophagus in northeastern Italy. J. Natl. Cancer Inst. 82:1407–1411.
Frank, H.K. 1991. Food contamination by ochratoxin A in Germany. IARC Sci. Publ. 115:77-81.
Fremy, J.M. and S. Dragacci. 1999. Mycotoxines et produits laitiers. In: Les mycotoxines dans l’alimentation. Evaluation et gestion du risque (A. Pfohl-Leszkowicz, ed). Editions TEC and DOC, Paris, pp. 353-369.
Frisvad, J.C. and U. Thrane. 1996. Mycotoxin production by foodborne fungi. In: Introduction to Food-borne Fungi (R.A. Samson, E.S. Hoekstra, J.C. Frisvad and O. Filtenborg, eds). Centraalbureau voor Schimmelcultures: Baarn, The Netherlands, 5th Edition, pp. 251-260.
Furlong, E.B., L.M.V. Soares, C.C. Lasca and E.Y. Kohara. 1995. Mycotoxins and fungi in wheat harvested during 1990 in test plots in the state of Säo Paulo, Brazil. Mycopathologia 131:185-190.
Galvano, F., V. Galofaro and G. Galvano. 1996. Occurrence and stability of aflatoxin M1 in milk and milk products. A worldwide review. J. Food Prot. 59:1079-1090.
Galvano, F., V. Galofaro, M. Bognanno, A. De Angelis and G. Galvano. 2001. Survey of the occurrence of aflatoxin M1 in dairy products marketed in Italy. Second year of observation. Food Addit. Contam. 18:644-646.
Galvano, F., V. Galofaro, A. De Angelis, M. Galvano, M. Bognanno and G. Galvano. 1998. Survey of the occurrence of aflatoxin M1 in dairy products marketed in Italy. J. Food Prot. 61:738-741.
Galvano, F., A. Ritieni, G. Piva and A. Pietri. 2005. Mycotoxins in the human food chain. In: Mycotoxin Blue Book (D. Diaz, ed). Nottingham University Press, UK, pp. 187-224.
Gareis, M., E. Martlbauer, J. Bauer and B. Gedek. 1988. Determination of ochratoxin A in human milk. Z. Lebensm. Unters. Forsch. 186:114-117.
Garrido, N.S., M.H. Iha, M.R. Santos Ortolani and R.M. Duarte Favaro. 2003. Occurrence of aflatoxins M(1) and M(2) in milk commercialized in Ribeirao Preto- SP., Brazil. Food Addit. Contam. 20:70-73.
Gelderblom, W.C.A., K. Jaskiewicz, W.F.O. Marasas, P.G. Thiel, R.M. Horak, R. Vleggaar and N.P.J. Kriek.
1988. Fumonisins - novel mycotoxins with cancerpromoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 54:1806- 1811.
Grabarkiewicz-Szczesna, J., E. Foremska and P. Golinski. 1996. Distribution of trichothecene mycotoxins in maize ears infected with F. graminearum and F. crookwellense. Mycotoxin Res. 12:45-50.
Grosso, F., S. Said, I. Mabrouk, J.M. Fremy, M. Castegnaro, M. Jemmali and S. Dragacci. 2003. New data on the occurrence of ochratoxin A in human sera from patients affected or not by renal diseases in Tunisia. Food Chem. Toxicol. 41:1133-1140.
Groves, F.D., L. Zhang, Y.S. Chang, P.F. Ross, H. Casper, W.P. Norred, W.C. You and J.F. Fraumeni, Jr. 1999. Fusarium mycotoxins in corn and corn products in a high-risk area for gastric cancer in Shandong Province, China. J. AOAC Int. 82:657-662.
Gutema, T., C. Munimbazi and L.B. Bullerman. 2000. Occurrence of fumonisins and moniliformin in corn and corn-based food products of U.S. origin. J. Food Prot. 63:1732-1737.
Hassen, W., S. Abid, A. Achour, E. Creppy and H. Bacha. 2004. Ochratoxin A and ß(2)- microglobulinuria in healthy individuals and in chronic interstitial nephropathy patients in the Centre of Tunisia: a hot spot of ochratoxin A exposure. Toxicology 199:185-193.
Hietaniemi, V. and J. Kumpulainen. 1991. Contents of Fusarium toxins in Finnish and imported grains and feeds. Food Addit. Contam. 8:171-182.
Hult, K. and R. Fuchs. 1986. Analysis and dynamics of ochratoxin A in biological systems. In: Mycotoxins and Phycotoxins (P.S. Steyn and R. Vleggaar, eds).
Elsevier Science Publishers BV, Amsterdam, pp. 365- 376.
IARC. 1993. IARC Monograph on the evaluation of carcinogenic risk to humans. Vol. 56: Toxins derived from F. moniliforme: Fumonisins B1 and B2 and fusarin C: In some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. IARC Press, Lyons, pp. 445- 466.
Jackson, L.S., T. Beacham-Bowden, S.E. Keller, C. Adhikari, K.T. Taylor, S.J. Chirtel and R.I. Merker. 2003. Apple quality, storage, and washing treatments affect patulin levels in apple cider. J. Food Prot. 66(4):618-624.
Jestoi, M., A. Ritieni and A. Rizzo. 2004. Analysis of the Fusarium mycotoxins fusaproliferin and trichothecenes in grains using gas chromatographymass spectrometry. J. Agric. Food Chem. 52:1464- 1469.
Jimenez, A.M., A. Lopez de Cerain, E. Gonzalez-Penas and J. Bello. 2001. Determination of ochratoxin A in pig liver-derived pates by high-performance liquid chromatography. Food Addit. Contam. 18:559-563.
Joint FAO/WHO Expert Committee on Food Additives (JECFA) 2001. Safety evaluation of certain mycotoxins in food. Prepared by the Fifty-sixth Meeting of JECFA. WHO food additives series 47/ FAOfood and nutrition 74-International Programme on Chemical Safety (IPCS). WHO, Geneva.
Jonsyn, F.E., S.M. Maxwell and R.G. Hendrickse. 1995. Ochratoxin A and aflatoxins in breast milk samples from Sierra Leone. Mycopathologia 131:121-126.
Jorgensen, K. 1998. Survey of pork, poultry, coffee, beer and pulses for ochratoxin A. Food Addit. Contam. 15:550-554.
Jorgensen, K. and A. Petersen. 2002. Content of ochratoxin A in paired kidney and meat samples from healthy Danish slaughter pigs. Food Addit. Contam. 19:562-567.
Julian, A.M., P.W. Wareing, S.I. Phillips, V.F.P. Medlock, M.V. MacDonald and L.E. del Rio. 1995.
Fungal contamination and selected mycotoxins in preand post-harvest maize in Honduras. Mycopathologia 129:5-16.
Kew, M.C. 2003. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 23:405-409.
Khalef, A., C. Zidane, A. Charef, A. Gharbi, M. Tadjerouna, A.M. Betbeder and E.E. Creppy. 1993.
Ochratoxicoses humaines en Algerie. In: Human Ochratoxicosis and its Pathologies, INSERM Vol 231 (E. Creppy, M. Castegnaro and G. Dirheimer, eds). John Libbey Eurotext Ltd, Paris, pp.123-127.
Kim, E.K., D.H. Shon, D. Ryu, J.W. Park, H.J. Hwang and Y.B. Kim. 2000. Occurrence of aflatoxin M1 in Korean dairy products determined by ELISA and HPLC. Food Addit. Contam. 17:59-64.
Kpodo, K., U. Thrane and B. Hald. 2000. Fusaria and fumonisins in maize from Ghana and their cooccurrence with aflatoxins. Int. J. Food Microbiol. 61:147-157.
Kuiper-Goodman, T. 1995. Mycotoxins: risk assessment and legislation. Toxicol. Ltr. 82-83:853-859. Kuiper-Goodman, T. 1996. Risk assessment of ochratoxin A: an update. Food Addit. Contam. 13(Suppl):53-7.
Langseth, W. and T. Rund berget. 1999. The occurrence of HT-2 toxin and other trichothecenes in Norwegian cereals. Mycopathologia 147(3):157-165.
Langseth, W. and O. Elen. 1996. Differences between barley, oats and wheat in the occurrence of deoxynivalenol and other trichothecenes in Norwegian grain. J. Phytopathol. 144:113-118.
Larsen, T.O., J.C. Frisvad, G. Ravn and T. Skaaning. 1998. Mycotoxin production by Penicillium expansum on blackcurrant and cherry juice. Food Addit. Contam. 15(6):671-675.
Lauren, D.R., D.J. Jensen, W.A. Smith, B.W. Dow and S.T. Sayer. 1996. Mycotoxins in New Zealand maize: a study of some factors influencing contamination levels in grain New Zealand. J. Crop Hortic. Sci. 24:13-20.
Leblanc, J.C., L. Malmauret, D. Delobel, P. Verger. 2002. Simulation of the exposure to deoxynivalenol of French consumers of organic and conventional foodstuffs. Regul. Toxicol. Pharmacol. 36:149-154.
Li, F.Q., Y.W. Li, X.Y. Luo and T. Yoshizawa. 2002. Fusarium toxins in wheat from an area in Henan Province, PR China, with a previous human red mould intoxication episode. Food Addit. Contam. 19:163- 167.
Maarou, K., A. Achour, A.M. Betbeder, M. Hamammi, F. Ellouz, E.E. Creppy and H. Bacha. 1995a. Foodstuffs and human blood contamination by the mycotoxin ochratoxin A: correlation with chronic interstitial nephropathy in Tunisia. Arch. Toxicol. 69:552-558.
Maarou, K., A. Achour, M. Hamammi, M. El May, A.M. Betbeder, F. Ellouz, E.E. Creppy and H. Bacha. 1995b. Ochratoxin A in human blood in relation to nephropathy in Tunisia. Human Exper. Toxicol. 14:609-615.
Manthey, F.A., C.E. Wolf-Hall, S. Yalla, C. Vijayakumar and D. Carlson. 2004. Microbial loads, mycotoxins, and quality of durum wheat from the 2001 harvest of the northern plains region of the United States. J. Food Prot. 67:772-780.
Marasas, W.F. 1997. Risk assessment of fumonisins produced by Fusarium moniliforme in corn. Cereal Res. Commun. 25:399-406.
Marasas, W.F. 2001. Discovery and occurrence of the fumonisins: a historical perspective. Environ. Health Perspect. 109:239-243.
Marasas, W.F., R.T. Riley, K.A. Hendricks, V.L. Stevens, T.W. Sadler, J. Gelineau-Van Waes, S.A. Missmer, J. Cabrera, O. Torres, W.C. Gelderblom, J. Allegood, C. Martinez, J. Maddox, J.D. Miller, L. Starr, M.C. Sullards, A.V. Roman, K.A. Voss, E. Wang and A.H. Merrill, Jr. 2004. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisincontaminated maize. J. Nutr. 134:711-716.
Marasas, W.F.O. 1995. Fumonisins: their implications for human and animal health. Natural Toxins 3:193- 198.
Martins, M.L., A. Gimeno, H.M. Martins and F. Bernardo. 2002. Co-occurrence of patulin and citrinin in Portuguese apples with rotten spots. Food Addit. Contam. 19:568-574.
Micco, C., M.A. Ambruzzi, M. Miraglia, C. Brera, R. Onori and L. Benelli. 1991. Contamination of human milk with ochratoxin A. IARC Sci. Publ. 115:105- 108.
Micco, C., C. Brera, M. Miraglia and R. Onori. 1987. HPLC determination of the total content of aflatoxins in naturally contaminated eggs in free and conjugated forms. Food Addit. Contam. 4:407-414.
Micco, C., M. Miraglia, C. Brera, S. Corneli and A. Ambruzzi. 1995. Evaluation of ochratoxin A level in human milk in Italy. Food Addit. Contam. 12:351- 354.
Miraglia, M., A. de Dominicis, C. Brera, S. Corneli, E. Cava, E. Menghetti and E. Miraglia. 1995. Ochratoxin A levels in human milk and related food samples: an exposure assessment. Nat. Toxins 3:436-444.
Mortimer, D.N., I. Parker, M.J. Shepherd and J. Gilbert. 1985. A limited survey of retail apple and grape juices for the mycotoxin patulin. Food Addit. Contam. 2:165-170.
Mousing, J., J. Kyrval, T.K. Jensen, B. Aalbaek, J. Buttenschon, B. Svensmark and P. Willeberg. 1997.
Meat safety consequences of implementing visual postmortem meat inspection procedures in Danish slaughter pigs. Vet. Rec. 140:472-477.
Muller, H.M. and K. Schwadorf. 1993. A survey of the natural occurrence of Fusarium toxins in wheat grown in a southwestern area of Germany. Mycopathologia 121:115-121.
Northolt, M.D., J.C. Frisvad and R.A. Samson. 1996. Occurrence of food-borne fungi and factors for growth. In: Introduction to Food-borne Fungi (R.A. Samson, E.S. Hoekstra, J.C. Frisvad and O. Filtenborg, eds). Centraalbureau voor Schimmelcultures: Baarn, The Netherlands, pp. 243- 250.
Ogundero, V.W. 1987. Crown-rot fungi of Nigerian bananas cv. Robusta and the effects of benomyl on their exoenzymes. J. Basic Microbiol. 27:43-47.
Oliveira, C.A., P.M. Germano, C. Bird and C.A. Pinto. 1997. Immunochemical assessment of aflatoxin M1 in milk powder consumed by infants in Säo Paulo, Brazil. Food Addit. Contam. 14:7-10.
Olsen, M., I. Thorup, I. Knudsen, J. Larsen, B. Hald and J. Olsen. 1991. In: Nordiske Seminar-og Arbejdsrapporter, Health Evaluation of Ochratoxin A in Food Products, Vol.545. Nordic Council of Ministers, Copenhagen.
Ono, E.Y., M.A. Ono, F.Y. Funo, A.E. Medinal, T.C. Oliveira, O. Kawamura, Y. Ueno and E.Y. Hirooka. 2001. Evaluation of fumonisin-aflatoxin cooccurrence in Brazilian corn hybrids by ELISA. Food Addit. Contam. 18:719-729.
Oruc, H.H. and S. Sonal. 2001. Determination of aflatoxin M1 levels in cheese and milk consumed in Bursa, Turkey. Vet. Hum. Toxicol. 43:292-293.
Otteneder, H. and P. Majerus. 2000. Occurrence of ochratoxin A in wines: influence of the type of wine and its geographical origin. Food Addit. Contam. 17:793-798.
Ough, C.S. and C.A. Corison. 1980. Measurement of patulin in grapes and wines. J. Food Sci. 43(3):476- 478.
Pacin, A.M., S.L. Resnik, M.S. Neira, G. Molto and E. Martinez. 1997. Natural occurrence of deoxynivalenol in wheat, wheat flour and bakery products in Argentina. Food Addit. Contam. 14:327-331.
Panigrahi, S. 1997. Alternaria toxins. In: Handbook of Plant and Fungal Toxicants (J.P.F. D’Mello, ed). CRC Press, Boca Raton, FL, USA, pp. 319-337.
Park, J.W., E.K. Kim and Y.B. Kim. 2004. Estimation of the daily exposure of Koreans to aflatoxin B1 through food consumption. Food Addit. Contam. 21:70-75.
Park, J.W., E.K. Kim, D.H. Shon and Y.B. Kim. 2002. Natural co-occurrence of aflatoxin B1, fumonisin B1 and ochratoxin A in barley and corn foods from Korea. Food Addit. Contam. 19:1073-1080.
Peraica, M., A.M. Domijan, M. Matasin, A. Lucic, B. Radic, F. Delas, M. Horvat, I. Bosanac, M. Balija and D. Grgicevic. 2001. Variations of ochratoxin A concentration in the blood of healthy populations in some Croatian cities. Arch. Toxicol. 75:410-414.
Perkowski,, J., R.D. Plattner, P. Golinski and R.F. Vesonder. 1990. Natural occurrence of deoxynivalenol, 3-acetyldeoxynivalenol, 15-acetyl-deoxynivalenol, nivalenol, 4,7-dideoxynivalenol and zearalenone in Polish wheat. Mycotoxin Res. 6:7-12.
Petersen, A. and I. Thorup. 2001. Preliminary evaluation of fumonisins by the Nordic countries and occurrence of fumonisins (FB1 and FB2) in corn-based foods on the Danish market. Food Addit. Contam. 18:221-226.
Petkova-Bocharova, T., I.N. Chernozemsky and M. Castegnaro. 2002. Balkan endemic nephropathy and associated urinary tract tumours: a review on aetiological causes and the potential role of mycotoxins. Food Addit. Contam. 19:282-302.
Pfohl-Leszkowicz, A., T. Petkova-Bocharova, I.N. Chernozemsky and M. Castegnaro. 2002. Balkan endemic nephropathy and associated urinary tract tumours: a review on aetiological causes and the potential role of mycotoxins. Food Addit. Contam. 19:282-302.
Pieters, M.N., J. Freijer, B.J. Baars, D.C. Fiolet, J. van Klaveren and W. Slob. 2002. Risk assessment of deoxynivalenol in food: concentration limits, exposure and effects. Adv. Exp. Med. Biol. 504:235-248.
Pietri, A., T. Bertuzzi, F. Gadolini and A. Gualla. 2001. Aflatoxin B1 residues in eggs of laying hens fed a naturally contaminated diet. Proceedings of the A.S.P.A. XIV Congress, Firenze, June 12-15, pp. 448- 450.
Pietri, A., T. Bertuzzi, M. Moschini and G. Piva. 2003. Aflatoxin M1 occurrence in milk samples destined for Parmigiano Reggiano Cheese production. Ital. J. Food Sci. 15:301-306.
Pietri, A., T. Bertuzzi, L. Pallaroni and G. Piva. 2001. Occurrence of ochratoxin A in Italian wines. Food Addit. Contam. 18:647-654.
Pietri, A., T. Bertuzzi, L. Pallaroni and G. Piva. 2004. Occurrence of mycotoxins and ergosterol in maize harvested over 5 years in Northern Italy. Food Addit. Contam. 21:479-487.
Pineiro, M.S., G.E. Silva, P.M. Scott, G.A. Lawrence and M.E. Stack. 1997. Fumonisin level in Uruguayan corn products. J. AOAC Int. 80:825-828.
Pitt, J.I. 2000. Toxigenic fungi: Which are important? Med. Mycol. 38(Supp. 1):17–22.
Placinta, C.M., J.F.P. d’Mello and A.M.C. Macdonald. 1999. A review of worldwide contamination of cereal grains and animal feeds with Fusarium mycotoxins. Anim. Feed Sci. Tech. 78:21-37.
Qiu, M., X. Liu, Y. Wang and C. Zhang. 2001. Survey on the fumonisin intake and the urinary Sa/So ratio of people suffering from a high incidence of esophageal cancer. Wei Sheng Yan Jiu. 30:365-367.
Rasmussen, P.H., F. Ghorbani and T. Berg. 2003. Deoxynivalenol and other Fusarium toxins in wheat and rye flours on the Danish market. Food Addit. Contam. 20:396-404.
Rastogi, S., P.D. Dwivedi, S.K. Khanna and M. Das. 2004. Detection of aflatoxin M1 contamination in milk and infant milk products from Indian markets by ELISA. Food Contr. 15:287-290.
Rheeder, J.P., E.W. Sydenham, W.F.O. Marasas, P.G. Thiel, G.S. Shephard, M. Schlechter, S. Stockenstrom, D.W. Cronje and J.H. Viljoen. 1995. Fungal infestation and mycotoxin contamination of South African commercial maize harvested in 1989 and 1990. S. Afr. J. Sci. 91:127-131.
Ritieni, A. 2003. Patulin in Italian commercial apple products. J. Agric. Food Chem. 51(20):6086-6090.
Ritieni, A., A. Moretti, A. Logrieco, A. Bottalico, G. Randazzo, S.M. Monti, R. Ferracane and V. Fogliano. 1997. Occurrence of fusaproliferin, fumonisin B1, and beauvericin in maize from Italy. J. Agric. Food Chem. 45:4011-4016.
Roussi, V., A. Govaris, A. Varagouli and N.A. Botsoglou. 2002. Occurrence of aflatoxin M1 in raw and market milk commercialized in Greece. Food Addit. Contam. 19:863-868.
Ryu, J.C., J.S. Yang, Y.S. Song, O.S. Kwon, J. Park and I.M. Chang. 1996. Survey of natural occurrence of trichothecene mycotoxins and zearalenone in Korean cereals harvested in 1992 using gas chromatography/mass spectrometry. Food Addit. Contam. 13:333-341.
Schollenberger, M., S. Suchy, H.T. Jara, W. Drochner and H.M. Muller. 1999. A survey of Fusarium toxins in cereal-based foods marketed in an area of southwest Germany. Mycopathologia 147:49-57.
Schothorst, R.C. and H.P. Van Egmond. 2004. Report from SCOOP task 3.2.10. Collection of occurrence data of Fusarium toxins in food and assessment of dietary intake by the population of EU member states: Subtask: trichothecenes. Toxicol Ltr. 153:133-143.
Schwartz, G.G. 2002. Hypothesis: does ochratoxin A cause testicular cancer? Cancer Causes Control. 13:91- 100.
Scientific Committee on Food Opinion on Ochratoxin A, CS/CNTM/MYC/14 final, Annex II to Document XXIV/2210/98, European Commission, Bruxelles, 17 September 1998.
Scott, P.M. and G.A. Lawrence. 1997. Determination of aflatoxins in beer. J. AOAC Int. 80:1229-1234.
Scott, P.M., W.F. Miles, P. Toft and J.G. Dube. 1972. Occurrence of patulin in apple juices. J. Ag. Food Chem. 20(2):450-451.
Scott, P.M. 1989. The natural occurrence of trichothecenes. In: Trichothecene Mycotoxicosis: Pathophysiologic Effects, Vol. I (V.R. Beasley, ed). CRC Press, Boca Raton, FL, USA, pp. 1-26.
Shenasi, M., K.E. Aidoo and A.A. Candlish. 2002. Microflora of date fruits and production of aflatoxins at various stages of maturation. Int. J. Food Microbiol. 79:113-119.
Shephard, G.S., W.F. Marasas, H. Yazdanpanah, H. Rahimian, N. Safavi, A. Zarghi, A. Shafaati and H.R. Rasekh. 2002. Fumonisin B1 in maize harvested in Iran during 1999. Food Addit. Contam. 19:676-679.
Shephard, G.S., N.L. Leggott, S. Stockenström, N.I.M. Somdyala and W.F.O. Marasas. 2002. Preparation of South African maize porridge: effect on fumonisin mycotoxin levels. South African J. Sci. 98:393-396.
Siame, B.A., S.F. Mpuchane, B.A. Gashe, J. Allotey and G. Teffera. 1998. Occurrence of aflatoxins, fumonisin B1, and zearalenone in foods and feeds in Botswana. J. Food Prot. 61:1670-1673.
Skaug, M.A. 1999. Analysis of Norwegian milk and infant formulas for ochratoxin A. Food Addit. Contam. 16:75-78.
Skaug, M.A., I. Helland, K. Solvoll, O.D. Saugstad. 2001. Presence of ochratoxin A in human milk in relation to dietary intake. Food Addit. Contam. 18:321-327.
Skaug, M.A., F.C. Stormer and O.D. Saugstad. 1998. Ochratoxin A: a naturally occurring mycotoxin found in human milk samples from Norway. Acta Paediatr. 87:1275-1278.
Smith, J.E., 1997. Aflatoxins. In: Handbook of Plant and Fungal Toxicants (J.P.F. D’Mello, ed). CRC Press, Boca Raton, FL, pp. 269-285.
Sohn, H.B., J.A. Seo and Y.W. Lee. 1999. Cooccurrence of Fusarium mycotoxins in mouldy and healthy corn from Korea. Food Addit. Contam. 16:153-158.
Speijers, G.J.A. and H.P. van Egmond. 1993. Worldwide ochratoxin A levels in food and feeds. In: Human ochratoxicosis and its pathologies, Vol. 231 (E.E. Creppy, M. Castegnaro, G. Dirheimer, eds). John Libbey Eurotoext Lt., Montrouge, pp. 85-100.
Srivastava, V.P., A. Bu-Abbas, Alaa-Basuny, W. Al- Johar, S. Al-Mufti and M.K. Siddiqui. 2001. Aflatoxin M1 contamination in commercial samples of milk and dairy products in Kuwait. Food Addit. Contam. 18:993-997.
Stefanaki, I., E. Foufa, A. Tsatsou-Dritsa and P. Dais. 2003. Ochratoxin A concentrations in Greek domestic wines and dried vine fruits. Food Addit. Contam. 20:4- 83.
Stinson, E.E., S.F. Osman, C.N. Huhtanen and D.D. Bills. 1978. Disappearance of patulin during alcoholic fermentation of apple juice. Appl. Envinron. Microbiol. 36(4):620-622.
Stoloff, L. 1980. Aflatoxin M1 in perspective. J. Food Prot. 43:226-230.
Stratton, G.W., A.R. Robinson, H.C. Smith, L. Kittilsen and M. Barbour. 1993. Levels of five mycotoxins in grains harvested in Atlantic Canada as measured by high performance liquid chromatography. Arch. Environ. Contam. Toxicol. 24:399-409.
Studer-Rohr, I., J. Schlatter and D.R. Dietrich. 2000 Kinetic parameters and intraindividual fluctuations of ochratoxin A plasma levels in humans. Arch. Toxicol. 74:499-510.
Sugiura,Y., K. Fukasaku, T. Tanaka, Y. Matsui and Y. Ueno. 1993. Fusarium poae and Fusarium crookwellense, fungi responsible for the natural occurrence of nivalenol in Hokkaido. Appl. Environ. Microbiol. 59:3334-3338.
Tanaka, T., S. Yamamoto, A. Hasegawa, N. Aoki, J.R. Besling, Y. Sugiura and Y. Ueno. 1990. A survey of the natural occurrence of Fusarium mycotoxins, deoxynivalenol, nivalenol and zearalenone, in cereals harvested in The Netherlands. Mycopathologia 110:19-22.
Trucksess, M.W., M.A. Dombrink-Kurtzman, V.H. Tournas and K.D. White. 2002. Occurrence of aflatoxins and fumonisins in Incaparina from Guatemala. Food Addit. Contam. 19:671-5.
Trucksess, M.W., M.E. Stack, S. Allen and N. Barrion. 1995b. Immunoaffinity column coupled with liquid chromatography for determination of fumonisin B1 in canned and frozen sweet corn. J. AOAC Int. 78:705- 710.
Trucksess, M.W., F. Thomas, K. Young, M.E. Stack, W.J. Fulgueras and S.W. Page. 1995a. Survey of deoxynivalenol in U.S. 1993 wheat and barley crops by enzyme-linked immunosorbent assay. J. AOAC Int. 78:631-636.
Tseng, T.C. and C.Y. Liu. 1997. Occurrence of fumonisin B1 and B2 in corn-based foodstuffs in Taiwan market. Mycopathologia 137:57-61.
Tseng, T.C. and C.Y. Liu. 1999. Natural occurrence of fumonisins B1 and B2 in domestic maize of Taiwan. J. Agric. Food Chem. 47:4799-4801.
Tseng, T.C. and C.Y. Liu. 2001. Occurrence of fumonisin B1 in maize imported into Taiwan. Int. J. Food Microbiol. 65:23-26.
van der Stegen, G.H.D., P.J.M. Essens and J. van der Lijn. 2001. Effect of roasting conditions on reduction of ochratoxin A in coffee. J. Agric. Food Chem. 49:4713-4715.
van der Westhuizen, L., G.S. Shephard, V.M. Scussel, L.L. Costa, H.F. Vismer, J.P. Rheeder and W.F. Marasas. 2003. Fumonisin contamination and Fusarium incidence in corn from Santa Catarina, Brazil. J. Agric. Food Chem. 51:5574-5578.
Vargas, E.A., R.A. Preis, L. Castro and C.M. Silva. 2001. Co-occurrence of aflatoxins B1, B2, G1, G2, zearalenone and fumonisin B1 in Brazilian corn. Food Addit. Contam. 18:981-986.
Verger, P., J.L. Volatier and A. Dufour. 1999. Estimation des niveaux théoriques d’ingestion d’aflatoxines et d’ochratoxine. In: Les mycotoxines dans l’alimentation: evaluation et gestion du risque (A. Pfohl-Leszkowicz, ed). TEC & DOC Lavoisier, Paris, France, pp. 371-384.
Vinas, I., J. Dadon and V. Sanchis. 1993. Citrininproducing capacity of Penicillium expansum strains from apple packing houses of Lerida (Spain). Int. J. Food Microbiol. 19(2):153-156.
Viquez, O.M., M.E. Castell-Perez and R.A. Shelby. 1996. Occurrence of fumonisin B1 in maize grown in Costa Rica. J. Agric. Food Chem. 44:2789-2791.
Visconti, A. and M.B. Doko. 1994. Survey of fumonisin production by Fusarium isolated from cereals in Europe. J. AOAC Int. 77:546-550.
Vrabcheva, T., R. Gessler, E. Usleber and E. Martlbauer. 1996. First survey on the natural occurrence of Fusarium mycotoxins in Bulgarian wheat. Mycopathologia 136:47-52.
Vrabcheva, T., T. Petkova-Bocharova, F. Grosso, I. Nikolov, I.N. Chernozemsky, M. Castegnaro and S. Dragacci. 2004. Analysis of ochratoxin A in foods consumed by inhabitants from an area with Balkan endemic nephropathy: a 1 month follow-up study. J. Agric. Food Chem. 52:2404-2410.
Vrabcheva, T., E. Usleber, R. Dietrich and E. Martlbauer. 2000. Co-occurrence of ochratoxin A and citrinin in cereals from Bulgarian villages with a history of Balkan endemic nephropathy. J. Agric. Food Chem. 48:2483-2488.
Wafa, E.W., R.S. Yahya, M.A. Sobh, I. Eraky, M. el- Baz, H.A. el-Gayar, A.M. Betbeder and E.E. Creppy. 1998. Human ochratoxicosis and nephropathy in Egypt: a preliminary study. Hum. Exp. Toxicol. 17:124-129.
Walker, R. 2002. Risk assessment of ochratoxin: current views of the European Scientific Committee on Food, the JECFA and the Codex Committee on Food Additives and Contaminants. Adv. Exp. Med. Biol. 504:249-255.
Wang, H., H. Wei, J. Ma and X. Luo. 2000. The fumonisin B1 content in corn from North China, a high-risk area of esophageal cancer. J. Environ. Pathol. Toxicol. Oncol. 19:139-141.
Wang, D.S., Y.X. Liang, N.T. Chau, L.D. Dien, T. Tanaka and Y. Ueno. 1995a. Natural co-occurrence of Fusarium toxins and aflatoxin B1 in corn for feed in North Vietnam. Nat. Toxins 3:445-449.
Wang, D.S., Y.X. Liang, K. Iijima, Y. Sugiura, T. Tanaka, G. Chen, S.Z. Yu and Y. Ueno. 1995b. Cocontamination of mycotoxins in corn harvested in Haimen, a high risk area of primary liver cancer in China. Mycotoxins 41:67-70.
WHO. 1998. Food Safety Issues. GEMS/FOOD Regional Diets.
WHO. 2001. Ochratoxin A. Safety evaluation of certain mycotoxins in food. International Program on Chemical Safety. WHO Food Additives Series (Vol. 47), World Health Organization, Geneva, pp. 281- 415.
WHO. 2002. Evaluation of certain mycotoxins in food. WHO Technical Report Series n. 906, World Health Organization, Geneva, pp. 8-42.
Wood, G.A., 1991. Aflatoxin M1. In: Mycotoxins and Phytoalexins (R.P. Sharma and D.K. Salunkhe, eds). CRC Press, Boca Raton, Florida, USA, pp.145-164.
Yamashita, A., T. Yoshizawa, Y. Aiura, P.C. Sanchez, E.I. Dizon, R.H. Arim and Sardjono. 1995. Fusarium mycotoxins (fumonisins, nivalenol and zearalenone) and aflatoxins in corn from southeast Asia. Biosci. Biotech. Biochem. 59:1804-1807.
Yoshizawa, T. 1991. Natural occurrence of mycotoxins in small grain cereals (wheat, barley, rye, oats, sorghum, millet, rice). In: Mycotoxins and Animal Foods (J.E. Smith and R.S. Henderson, eds). CRC Press, Boca Raton, Florida, USA, pp. 301-324.
Yoshizawa, T. and Y.Z. Jin. 1995. Natural occurrence of acetylated derivatives of deoxynivalenol and nivalenol in wheat and barley in Japan. Food Addit. Contam. 12:689-694.
Yoshizawa, T., A. Yamashita and N. Chokethaworn. 1996. Occurrence of fumonisins and aflatoxins in corn from Thailand. Food Addit. Contam. 13:163-168.
Yoshizawa, T., A. Yamashita and Y. Luo. 1994. Fumonisin occurrence in corn from high- and lowrisk areas for human esophageal cancer in China. Appl. Environ. Microbiol. 60:1626-1629.
Yousef, A.E. and E.H. Marth. 1989. Stability and degradation of Aflatoxin M1. In: Mycotoxins in Dairy Products (H.P. Van Egmond, ed). Elsevier Applied Science, London and New York, pp. 11-55.
Zimmerli, B. and R. Dick. 1995. Determination of ochratoxin A at the ppt level in human blood, serum, milk and some foodstuffs by high-performance liquid chromatography with enhanced fluorescence detection and immunoaffinity column cleanup: methodology and Swiss data. J. Chromatogr. B. Biomed. Appl. 666:85-99.
Zimmerli, B. and R. Dick. 1996. Ochratoxin A in table wine and grape-juice: occurrence and risk assessment. Food Addit. Contam. 13:655-668.
Authors: FABIO GALVANO1, ALBERTO RITIENI2, ANTONINO DE LORENZO3, GIANFRANCO PIVA4 and AMEDEO PIETRI4
1Department of Agro-forestry and Environmental Science, Mediterranean University of Reggio Calabria, Italy
2Department of Food Science, Federico II University, Napoli, Italy
3Department of Neuroscience, University of Tor Vergata, Rome
4Institute of Food Science and Nutrition, Catholic University of the Sacred Heart, Piacenza, Italy










