Breeding for resistance to ear rots caused by Fusarium spp. in maize – a review
Published: March 20, 2014
By: Ákos Mesterházy (Cereal Research Non-profit Company), Marc Lemmens (University for Natural Resources and Life Sciences) and Lana M. Reid (Eastern Cereal and Oilseed Research Centre)
Plant Breeding 131, 1—19 (2012) doi:10.1111/j.1439-0523.2011.01936.x
2011 Blackwell Verlag GmbH
2011 Blackwell Verlag GmbH
Received February 17, 2011/Accepted November 7, 2011
Communicated by T. Miedaner
Communicated by T. Miedaner
Abstract
Ear rots caused by different Fusarium spp. are one of the most dangerous food and feed safety challenges in maize production. At present, the majority of the inbreds and hybrids are susceptible. Gibberella and Fusarium ear rots (caused by Fusarium graminearum and Fusarium verticillioides, respectively) are the two main diseases, but more than 10 further Fusarium spp. cause ear rots. Natural infection is initiated by a mixture of the local Fusarium spp., but usually one species predominates. Many maize breeders rely on natural infection to create sufficient levels of disease severity for selection-resistant genotypes; however, there are few locations where the natural infection is sufficiently uniform to make this selection efficient and successful. Thus, an artificial inoculation method normally performed with one fungal species is now used by more breeders. Most published papers on breeding for ear rot resistance are focused on either F. graminearum or F. verticillioides, and reports involving both or more Fusarium spp. are rare. Several reports support the hypothesis that resistance to multiple species especially F. graminearum, F. culmorum and F. verticillioides may be common. Significant differences in genotypic resistance after inoculation exist. Resistance to the two major modes of fungal entry into the ear, via the silk or through kernel wounds, is not correlated in all genotypes. The reason is not clear. When silk channel resistance was assessed, the data from natural and artificial inoculation trials correlated well. Analogous data relating to kernel resistance have not been published. Both native and exotic sources of resistance are important, but surprisingly little information is available. Few papers report on the use of artificial inoculation during inbred development. Most of the publications on inoculation are concerned with testing at later stages when combining ability is tested. Inbreds differ in general and specific combining ability for ear rot resistance. The expression of resistance to disease severity and resistance to toxins is often used as synonyms, but in fact they are not. Higher resistance to visual disease severities mostly results in lower toxin contamination, and the resistance level seems to be the most important factor regulating the toxin content. The mode of inheritance of resistance appears to differ: additive, possibly non-additive effects, digenic (dominant) and polygenic patterns have been identified. Improved phenotyping methods that take into account the influence of stalk rot and the use of several independent isolates are available. The QTLs mostly exhibit small effects and some are validated; however, marker-assisted selection in breeding cannot yet be foreseen. As the severity of natural infections tends to correlate with the artificial inoculation results, the incorporation of artificial inoculation methods in breeding programmes is now the most important task. As genotypic resistance differences between hybrids are high, the registration of hybrids should consider the use of the inoculation tests to choose most resistant hybrids for commercial production. This is the most rapid way to increase feed safety.
Key words: Fusarium graminearum — Fusarium verticillioides — Fusarium — inoculation methods — resistance — inheritance of resistance—mycotoxins—breeding—corn—maize — Zea mays — ear rot.
Ear rot diseases of maize, or corn, caused by Fusarium spp. have long been known. One of the first scientific reports was that of Bisby and Bailey (1923) in Canada. In contrast to Fusarium stalk rots that often result in direct yield losses, ear rots rarely do so; however, occasional high yield losses have been reported (Vigier et al. 2001). As a consequence, only sporadic breeding efforts have been undertaken to increase resistance to ear rots. It was not until the discovery of Fusarium mycotoxins that the full impact of the indirect economic loss from an ear rot outbreak became known. New regulations for the allowable mycotoxin limits in food and feed have been put in place in most countries. Today, more and more maize breeding programmes at both public and private institutions are initiating and expanding breeding programmes to develop resistant inbreds and hybrids for both human and animal consumption.
In the 1920s and 1930s, hybrid breeding of maize was developed in the USA. By the late 1950s, hybrid varieties of maize dominated the maize acreage (Sprague 1977). This resulted in two significant changes in maize breeding: (i) the use of open pollinated landraces and varieties with their broader genetic diversity decreased and (ii) inbreeding for line and hybrid development was carried out in the same location, and owing to a lack of continuous epidemics, selection for ear rot resistance was not possible and susceptibility levels increased. As a result, sporadic resistance breeding programmes were initiated in North America and elsewhere. Initial studies were mostly on Gibberella ear rot (GER) caused by Fusarium graminearum Schwabe [teleomorph = Gibberella zeae (Schwein.) Petch]. Gibberella ear rot is not to be confused with Fusarium ear rot (FER) that is caused by a different Fusarium species, Fusarium moniliforme, which was recently reclassified by Seifert et al. (2004) as Fusarium verticillioides [=F. moniliforme J. Sheld. (sexual stage: G. moniliformis Wineland)]. Early on, it was reported that resistance to both diseases is inherited in a quantitative manner (Boling and Grogan 1965, Ullstrup 1977).
Interest in increasing ear rot resistance spiked with the detection of Fusarium mycotoxins in the grain. Zearalenone (ZEA) was the first toxin to be identified followed by deoxynivalenol (DON) (Mirocha 1974, Vesonder et al. 1979), both produced by F. graminearum. The F. verticillioides mycotoxin, fumonisin B1, was discovered in 1988 (Gelderblom et al. 1988). The main goal of breeding efforts was to develop either toxin resistance or disease resistance; however, the relationship between these two types of resistance was not clearly defined and they were often used as synonyms (Clements and White 2004, Menkir et al. 2006, Williams 2006). Knowledge relating to mycotoxins grew rapidly from many different laboratories. The metabolic synthesis and physiological effects on humans and livestock are now known for most of the toxins. This resulted in the establishment of legally allowable limits for many toxins in all important grain-producing regions of the world. Unfortunately, it did not result in the development of commercial hybrids with improved resistance as most are susceptible or very susceptible (Munkvold 2003b, Reinprecht et al. 2008).
Table 1. Fusarium spp. found in naturally infected maize grains in several maize-producing countries
As an alternative to or in addition to resistance breeding, breeding transgenic maize for increased insect resistance and better agronomic practices are suggested for ear rot control. Insect wounds, especially to the ear, from pests such as Ostrinia nubilalis, Diatraea grandiosella, Diabrotica virgifera virgifera, Heliocoverpa zeae and Frankliniella spp., increase the levels of Fusarium infection by creating new points of entry for the fungus to enter the plant (Archer et al. 2001, Parsons and Munkvold 2010a). Numerous recent articles have indicated that transgenic plants modified by Bt genes (genes from Bacillus thuringiensis with insecticidal properties) have significantly decreased DON and other toxins levels (Munkvold et al. 1997a, Dowd 2001, Munkvold 2003a, de la Campa et al. 2005, Papst et al. 2005, Wu 2006). No fungicide control has yet to be released until just recently in Europe (Lo¨ ffler et al. 2010a). Folcher et al. (2009) reported a 90% reduction in Fusarium mycoflora with some fungicides; however, further analysis is required.
Resistance to ear rots is important for both grain and silage maize. Stalk rot resistance in silage maize is a crucial trait, as mycotoxins can be found in stalks, but a detailed evaluation of stalk resistance is beyond the scope of this paper. For more information, the reader is referred to Christensen and Wilcoxson (1966) who published an excellent review of the early literature. More recent partial reviews can be found in Afolabi et al. (2008), Barrie`re et al. (1997), Nagy and Cabulea (1996), and Pappelis (1984); however, a comprehensive review of the past 50 years research has yet to be written.
The more recent binding EU regulations on toxin contamination for human consumption and recommendations for animal feeding (Anonymous 2005, 2006, 2007) have forced a renewed interest in breeding efforts for ear rot resistance as the preferred method of control. As the growing knowledge on resistance has not been reviewed in detail in the last few decades and numerous papers on resistance have been published since the two partial reviews by Munkvold (2003b) and Clements and White (2004), we decided to summarize the novel results and concepts developed and discuss several important conclusions for the future.
The Pathogens and Associated Mycotoxins The pathogens
One of the first reports on ear rots caused by Fusarium spp. originated from Canada by Bisby and Bailey (1923). The ear rot caused by F. graminearum was later called GER and is widespread in Canada, the USA and many other countries, Table 1 (Koehler 1957, 1959, Sutton and Baliko 1981, Reid et al. 1996a). Fusarium verticilliodes (referred to as F. moniliforme in papers published prior to the reclassification by Seifert et al. (2004) as F. verticillioides) was identified as a weak ear rot pathogen in the earlier reports (Ullstrup 1953, cited by Jugenheimer 1976) but has since been shown to be more of an endophyte fungi that tends to have lower visible symptoms of infection in the kernels and can be systemic in the maize plant (Munkvold et al. 1997a,b). This pathogen is the causal agent of FER. Other members of the Liseola Section like Fusarium proliferatum are also understood to be pathogens responsible for FER. Many different species occur in maize grain besides these three (Table 1), and in many cases, they play an important role in causing GER and FER. Folcher et al. (2009) identified 12 Fusarium spp. in France. In the southern regions, mainly F. verticillioides and F. proliferatum were present, and in the northern part, mainly F. graminearum and Fusarium culmorum. Hungary is similar; Mesterha´ zy and Vojtovics (1977) found that in dry years the distribution of the Fusarium spp. is closer to the distribution of Fusarium spp. in the south, and in wetter years the Fusarium spp. of the northern regions predominated. Other Hungarian results (Be´ke´ si and Hinfner 1970, Bi´ro´ ne´ 1975) support this complexity of the pathogen population. Similar species distributions are found in North America with GER predominantly found in the northern regions and FER in the southern regions or in dryer years in a northern area (Reid et al. 1999). A single ear or grain can occasionally be infected by different Fusarium spp. depending on the conditions of a growing season in a region (Logrieco et al. 2002). Identification of Fusarium spp. is easier than it used to be. The most important species have speciesspecific markers and can be quantified by PCR analysis (Reid et al. 1999, Pauls et al. 2001, Waalwijk et al. 2004, Xu et al. 2008, Nicolaisen et al. 2009).
Table 2: Mycotoxigenic Fusarium species associated with cereal crops and their mycotoxins (Logrieco et al. 2002)
Pathogenicity between Fusarium spp. and aggressiveness within a species is quite variable. Isolates of F. graminearum and F. culmorum are commonly highly aggressive with a considerable proportion of the isolates considered very aggressive. Fusarium verticillioides and the other Fusarium spp. tend to display lower aggressiveness. Careful screening of isolates is necessary to select the more aggressive ones for artificial inoculation in a breeding programme (Mesterha´ zy 1978, Reid et al. 2002, Iglesias et al. 2010, Miedaner et al. 2010). For F. graminearum and F. culmorum from wheat, Mesterha´ zy (1983, 1995, 2002) and Mesterha´ zy et al. (1999) found large variation in aggressiveness and DON production between isolates and years. Considerable differences in aggressiveness and toxin production were observed also when inocula from the same test tube were tested, and this did not correlate with the conidium concentrations measured. Similar data were obtained for maize (A´ . Mesterha´ zy, unpublished). Garcia et al. (2009) concluded that it would be difficult to predict mycotoxin levels because these levels are highly associated with the contaminating fungal strain not just the environmental conditions. Mari´n et al. (2008) also reported high variability in mycotoxin production between different fungal strains and on different substrates. Therefore, complicating prediction models based on visible symptoms and mycotoxin formation may be difficult to create. From the same isolate, inocula with different aggressiveness can be produced (Kova´ cs et al. 1994). For this reason, a change of inoculum, even though originating from one and the same isolate, within an experiment is not recommended as the two levels of aggressiveness of the different inocula can vary and influence infection levels. Therefore, each experiment is so planned that a change of inoculum should not occur. Further research on aggressiveness, fungal mass and conidium concentration is necessary.
Fusarium graminearum possesses different pathogenesisrelated (PR) genes (Dufresne et al. 2008), but these are not necessarily virulence genes and they cannot be used in a breeding programme. It is certain that Fusarium spp. have virulence factors, but it is not known whether the different Fusarium spp. possess common and/or different virulence factors. Mesterha´ zy et al. (1999) reported that DON production is a virulence factor of F. graminearum in wheat. Harris et al. (2005) also proved this for F. graminearum in maize; its role, however, is not known. Another risk factor may be the change in pathogenic population structure when more resistant hybrids are grown. No reliable information is available on this topic, but it will presumably significantly influence breeding programmes.
Although no data exist for maize, in wheat there is no evidence of race-specific specialization within Fusarium spp. (Snijders and van Eeuwijk 1991, Mesterha´ zy 1995). The same QTLs gave protection to all Fusarium spp. tested (Mesterha´ zy et al. 2007). Research in this field for maize should be undertaken. For resistance breeding, the appreciable number of Fusarium spp. creates a serious challenge.
Mycotoxins
Fusarium spp. produce a large number of chemically very different mycotoxins (Table 2) (Logrieco et al. 2002). The high diversity of these toxins and those detected recently (Barto´ k et al. 2006, 2010) excludes the possibility of selecting for general toxin resistance. The two toxins of most importance to GER are DON and ZEA. If contaminated grain is fed to livestock, especially swine, the trichothecene DON results in vomiting, feed refusal, decreased weight gain and reproductive problems (Vesonder et al. 1981, Prelusky et al. 1994). Storage workers might also be exposed to toxins, mainly through powder of infected grains in the air through inhalation, but this problem was not mentioned in the monograph of Christensen (1982). In the authorized Hungarian regulation, there is no word on this problem. We think that this research branch will be important in the future to see clear and make the necessary regulations. This toxin is also an immunosuppressant and thus predisposes animals to other diseases and masks underlying toxicoses (Pestka and Bondy 1994). Zearalenone causes reproductive problems including reduced litter size, swine oestrogenic syndrome and male infertility (Prelusky et al. 1994). Human relations are also important, immunsuppression of trichethecenes was proved (Berek et al. 2001), and ZEA influences also children hormone household causing telarche (Szu¨ ts et al. 1997). Therefore toxin contaminated grains may cause human health problems. FER results in the contamination of the grain with the polyketide fumonisin mycotoxins such as FB1, which causes equine leukoencephalomalacia (Kellerman et al. 1990), porcine pulmonary oedema (Harrison et al. 1990), liver cancer in rats (Gelderblom et al. 1988) and neural tube defects in mice (Voss et al. 2006). Fumonisins have also been associated with human oesophageal cancer (IARC 1993). Besides causing direct and indirect economic losses, these fungi can also affect the health of grain handlers and processors. Throughout the literature, resistance to these toxins and resistance to disease symptoms have been treated as two different types of resistance.
Some Fusarium spp. with apparent lower aggressiveness, as measured by visible disease symptoms, pose an additional challenge as these pathogens may be excellent toxin producers like F. verticillioides with its many fumonisin toxins and Fusarium sporotrichioides with its T-2 toxin. Resistance to these pathogens should therefore not be neglected. Resistance to one species may not be correlated to resistance to other species (Reid et al. 2002, 2009). Also, selection for resistance to a pure isolate of one species may not result in resistance to a multi-Fusarium population in commercial maize fields.
Infection Pathways, Disease Symptoms and Evaluation of Resistance
There are three main modes of fungal entry or infection pathways, by which Fusarium spp. enter maize ears: (i) by fungal spores landing on the silks, germinating and then the fungal mycelia grow down the silks to infect the kernels and cob (rachis) (Koehler 1942); (ii) wounds created by insects feeding on the ear or from bird or hail damage offer a point of entry for fungi (Sutton 1982); and (iii) some Fusarium spp. are systemic, such as F. verticilliodes, and can enter the ear from infected stalks (Foley 1959, Munkvold et al. 1997b). Which infection pathway is more important depends on the Fusarium spp. that is predominant and insect pressures in a given geographical location. In some locations, ear rot outbreaks are mainly associated with infection through the silk while in other locations where maize boring insects are a problem and are not controlled by other measures, infection through the kernels is predominant.
Gibberella ear rot
The symptoms of GER, caused mainly by F. graminearum, are characterized by a pinkish coloured mould (White 1999). Similar symptoms are found with F. culmorum infections; this species is a pathogen also associated with GER, but it lacks a Gibberella teleomorph. Infection of the ear commonly begins as white mycelium moving down from the ear tip. This mycelium later turns reddish-pink on infected kernels. In some cases, pinkish fungal growth can be found on the exterior husk leaves, and in severe infections, it is impossible to separate the husks from the kernels as the entire ear becomes a tightly bound mass of fungal and plant tissue that appears mummified. If infection occurred through kernel wounds, similar fungal growth is seen but it starts from the initial wound site and tends to spread to the tip of the ear faster than to the butt of the ear. The latter occurs because silks from the butt kernels emerge from the ear earlier than kernels from the tip of the ear and are hence pollinated earlier and dry earlier (Reid and Sinha 1998) Once the kernels reach 22– 23% moisture, it is difficult for the fungus to further infect (Christensen and Kaufmann 1969); however cob (rachis) moisture can be 15–25% higher than kernel moisture, so the infection may spread in the cobs and can enter younger kernels via the pedicel. In some cases, only the cob is infected; the ear may appear to be symptomless but when squeezed by hand, it will be quite spongy feeling and the cob will be wet and often pink/red in colour. How fast symptoms develop in a given year is highly dependent on the environment that influences not only ear development and subsequent kernel drydown but also fungal growth. The optimum temperature forGER development is 26– 28C, while FER rot has broader range extending to higher temperatures (Reid et al. 1999). GER also requires a much longer period of precipitation after infection usually around the time of plant (Reid et al. 1996a). Infection through the silks cannot proceed once the silks have dried out (Reid et al. 1992a, Reid and Sinha 1998). Recently, Xiang et al. (2010a) reported that there is a direct relationship between kernel drydown rates of a given maize genotype and the extent of ear rot severity symptoms. Husk tightness (Koehler 1959), ear declination and physiological resistance mechanisms all influence the spread of infection. Stalk rot and ear rot are strongly interrelated (Mesterha´ zy 1983, Mesterha´ zy and Kova´ cs 1988, Mesterha´ zy et al. 2000) as stalk rot interrupts the water supply to the ears and speeds up development and drying of the ear. This can reduce ear rot by 50% or more. Thus, it is important that in ear rot breeding nurseries, like all disease nurseries, the plants be as healthy as possible prior to ear rot inoculation; stalk rot should be controlled and only ear rot data on healthy stalks should be accepted to estimate resistance levels (Mesterha´ zy 1979, Kova´ cs et al. 1994).
Fusarium ear rot
In contrast to GER, symptoms of FER from F. verticillioides infection occur mainly on individual kernels or on limited areas of the ear (White 1999). In some ears, many independent infection sites may develop. Infected kernels develop a cottony growth or may develop white streaks on the pericarp and fungal growth on the cob. Ears infested by earworms are usually infected with F. verticillioides. Eller et al. (2008a) state that the disease is prevalent in warm, dry conditions, like those common to the southern United States, and F. verticillioides is found in grain or crop residue of virtually all mature maize fields in the United States. However, F. proliferatum and F. subglutinans are also minor causal agents of FER, as are probably other members of the Liseola Section of Fusaria. (Iglesias et al. 2010). Reid et al. (1996a) adds that rainy weather or irrigation at silking thereafter significantly increases disease severity for FER and especially for GER. Duncan and Howard (2010) studied the initial phases of the infection process by F. verticillioides and described in detail how the hyphae spread and colonize host cells and how the macroscopic symptoms develop. In many cases, the extent of toxin contamination is proportional to the visual severity of infection; however, asymptomatic kernels may also be infected and may contain toxins, usually trace amounts (Reid et al. 2009). Bacon and Hinton (1996) reported on the endophytic colonization of maize plants by F. moniliforme (F. verticillioides) and presumed that this can contribute to toxin contamination of the plants. Murillo-Williams and Munkvold (2008) reported that systemic infections from the stalk to the ear leading to more asymptomatic infections and toxin accumulation may be a problem predominantly in hot regions but not in the cooler areas. As global warming seems to be durable, this problem may become more serious in the currently cooler regions like central Europe and Canada.
Other ear rots
For the remaining Fusarium spp., a clear demonstration of the significance of a given species, its symptoms and epidemiological conditions is poorly documented. White (1999) does not even mention them. Their toxins, however, play a significant role; thus, their neglect is by no means justified. For example, T-2 toxin is produced by Fusarium sporotrichioides (Table 1) and others. As we have only FER and GER to describe these ear rots, a new term should be found for ear rots caused by other Fusarium spp. Perhaps, they can be grouped under the term Other Fusarium Ear Rot (OFER). It is unpractical to assign a new term to each of the species unless it is found that the incidence of a given species increases because of environmental or agronomic changes.
Reid et al. (1996a, 1999) stress that the two main Fusarium spp. may produce mixed infections. Some species will dominate in a field (Table 1) because of environmental conditions, but natural infection is almost always mixed. This makes breeding for resistance complicated as minor species should also be considered. Visual identification of a pathogen is often not possible: with mixed infections, atypical symptoms can occur. In inoculated trials, the breeder is determining which species is the predominant one; in trials where multiple species are inoculated at the same time, the environmental conditions after inoculation determine which species will predominate (Reid et al. 2001a,b). When natural infections are evaluated, general ear rot values based on visual symptoms are given without species identification. Most breeders follow this procedure, and Variety Offices like that in Hungary similarly report disease severity (percentage or rating of diseased area on the ear) and incidence (percentage of the number of visually infected ears) because of Fusarium spp. without species identification (Gergely et al. 2010).
Seedling blight
A greenhouse method was evaluated to check seedling stage resistance (Mesterha´ zy 1982). Commercial garden perlite (a very light-heated volcanic stone) is used, and this makes possible the washing of the whole plant and checking of plant damage and root rot more precisely. The method is used also for testing seed dresser against Fusarium seedling blight (Mesterha´ zy 1982).
Evaluation of disease
Disease incidence is measured as the number or percentage of visually infected ears. Disease severity is often rated visually on scales ranging from 1 to 5, 1 to 7 and 0 to 9 or as percentage of the ear surface with symptomatic kernels. For breeding purposes, the rating scales are commonly used, but for scientific studies the percentage of infected kernels may give more precise data. In addition to disease incidence and severity, it is important to assess other natural infections (e.g. Aspergillus) as well as the occurrence of insect, bird, wind or hail damage on the ear because all of these factors will influence infection levels. As wounds are often points of fungal entry, extensive damage may influence resistance measurements. In silk resistance evaluations, only ears without insect or other wound-mediated infection should be considered for evaluation and toxin analyses.
Reid and Sinha (1998) found that visual symptoms of GER stabilized and reached a maximum 6–8 weeks after inoculation of the silk channels. The rate of fungal spread after inoculation is quite variable and highly related to genotypic resistance. Mesterha´ zy et al. (2000) measured a fungal spread of 0.1–0.3% of the ear surface per day in highly resistant genotypes compared to 2–3% per day for the most susceptible genotypes measured after inoculation and until kernel moisture reached 28%. Similar to GER, working with FER Bush et al. (2004) found that fumonisin content is at a maximum at 20% kernel moisture and cannot be detected earlier than 35–40% moisture content. All of these studies indicate that for both GER and FER, symptoms reach a maximum 7 weeks after pollination and that toxin levels reach a maximum at 9 weeks. The later is therefore an optimum harvest time for ear rot evaluations; however, it should be noted that a wet fall and/or wet conditions in the grain after harvest can lead to more toxin development.
Considerable debate exists whether or not toxin evaluations should also be used to evaluate disease resistance. Without a doubt, the level of mycotoxins is an extremely important trait to record; however, mycotoxin analyses can be cost-prohibitive in some breeding programmes. Many breeders rely on visual ratings of disease severity for most of the inbred development and use toxin analysis for parent selection, testcross evaluation and the final developmental stages of inbreds and hybrids. It must be recognized that although there are published reports on high correlations between disease symptoms and toxin levels (Reid et al. 1996b) for F. graminearum, it seems to be necessary for F. verticillioides where significant differences in amount of toxins can be detected at the same visual severity rating (Butron et al. 2006). In their review relating to the prediction of mycotoxins in food, Garcia et al. (2009) state that not all fungal growth results in mycotoxin formation and the detection of mycotoxic fungi may or may not imply the presence of mycotoxins. Strains of mycotoxigenic species are able to synthesize mycotoxins in different amounts, and conditions conducive to fungal growth may not be conducive to mycotoxin production. For this reason, special attention must be paid to the relationship between visual symptoms and the level of toxin contamination, the NIRS may provide a cheap and efficient way to check toxin content.
Resistance Components and Artificial Inoculation
As the severity of natural infection is not consistent from year to year, maize researchers must use artificial inoculation methods to inoculate the plant material with fungal spores (Schaafsma et al. 1997). Currently, the only way to screen for resistance to GER is in the field. Satisfactory levels of infection and reliable genotypic differentiation have not been achieved under greenhouse conditions, and there is no laboratory technique or seedling test that can be used to screen for resistance that is exhibited in a fully grown plant (Reid et al. 1996a).
At present, the literature highlights the silk channel method of inoculation rather than the kernel wound inoculation by colonized toothpicks or injection of a fungal spore suspension. Both techniques result in the spread of infection from infected kernels to neighbouring kernels; however, with the silk channel inoculation, the infection must first proceed down the silks to the kernels. Whichever technique is used, the research is cautioned to first determine the major mode of fungal entry for the Fusarium spp. of interest in a given geographical area. The methods used for artificial inoculation and the evaluation of resistance are similar for both GER and FER as well as other Fusarium spp. Researchers working with two or more Fusarium spp. used the same methodology for all (Reid et al. 2002, 2009, Lo¨ ffler et al. 2010a).
Methods of inoculation
A number of artificial inoculation methods and their variants have been developed. They were first assessed by Ullstrup (1970). The oldest is the toothpick method of Young (1943). Working with this method, several improvements were made (Mesterha´ zy 1982, 1983). The toothpicks are boiled in deionized water three times to wash out tannins and other fungal growth–inhibiting compounds from the wood. Thereafter, they are air-dried and submerged into a suitable liquid medium (e.g. Czapek-Dox) for 1 h in an Erlenmeyer flask. Most of the medium is then removed leaving only 5–10 mm depth of media in the flask to ensure high humidity; the flask is then autoclaved for 1 h at 120C. A small amount of mycelium is transferred to the sterilized flask, and after 3 weeks the fungus grows through the toothpicks that are now ready to use (Mesterha´ zy 1982, 1983). The toothpicks are generally used for the inoculation of ears in two ways: insertion into the centre of the ear or into the silk channel representing kernel resistance or silk channel (silk) resistance, respectively (Reid et al. 1996a, Plienegger and Lemmens 2002). After 7–9 weeks, disease severity is estimated by the percentage of visually infected kernels directly or by using a rating scale as discussed earlier (Mesterha´ zy 1978, Enerson and Hunter 1980, Reid et al. 1996a). One of the disadvantages of this technique for silk channel resistance evaluation is with maize genotypes where the cob outgrows the husk leaves, causing the toothpick to fall out thus reducing infection severity. Another criticism of the toothpick technique is that infection levels can be too high because of the fact that the toothpick itself is a substrate for fungal growth and kernel inoculations result in severe wounding of several kernels and the cob (rachis).
It is important to choose an inoculation technique that results in a sufficient level of infection to differentiate genotypic differences in resistance but not so severe of an inoculation that these differences are hard to observe. It is rare that natural inoculations are as severe as artificial inoculations. As a consequence, several other methods of inoculation have been developed since the toothpick technique; however, it should be noted that for some researchers, the toothpick technique is necessary to achieve the best genotypic differentiation. Many researchers produce Fusarium suspensions in liquid media with or without aeration with sterile air, or the Fusarium conidia are washed from solid media poured into Petri dishes. The production methods are very variable (Mesterha´ zy 1978, Reid et al. 1996a, Plienegger and Lemmens 2002). Mesterha´ zy (1983) used the bubble breeding method that allowed the facile production of large quantities. If a researcher desires to inoculate with a mix of isolates or species, to avoid competition in the media, the isolates/species are grown separately and then just prior to inoculation mixed (Reid et al. 1996a, Presello et al. 2006). Lo¨ ffler et al. (2010a) used this method, but the isolates were not mixed to avoid isolate x isolate interactions. Conidia have been shown to be as good infection materials as mycelium (Takegami and Sasai 1970) and in fact, in natural infections, conidia are often the source of infection. The developed conidial suspension can be used in different ways (Papst et al. 2007): the silks can be sprayed with the suspension, or the cob tip can be immersed into the suspension. The resistance type observed after this type of inoculation is termed silk resistance. After spraying, the silks can be covered with polyethylene bags to achieve higher humidity and consequently higher disease severity. However, the toothpick method gave more than double level of GER and FER severity than these methods (A´ . Mesterha´ zy and E. Toldi, unpublished) and covering the ear with a polyethylene bag also serves to increase the development of other fungi and bacteria, which may complicate resistance evaluations (L. M. Reid, unpublished). A more common use of the suspension is to inject it into the silk channel (silk channel resistance) or the centre of the ear (kernel resistance) using syringes or vaccinators (Reid et al. 1996a). The amount of inoculum injected can vary from several ll to 5 ml, with the larger volumes used for silk or silk channel resistance. Reid et al. (1996a, 2009) and Presello et al. (2008), for example, used 2 ml. Lo¨ ffler et al. (2010a) utilized only 1 ml. The conidium concentration varies: Presello et al. (2008) and Lo¨ ffler et al. (2010a) used 1 x 106 conidia/ml for FER, while Lo¨ ffler et al. (2010a) used 1 x 105 for GER. Clements et al. (2003) compared several inoculation methods for FER: injection of inoculum through the ear husk leaves at the R2 developmental stage; spray inoculation with different variants (coverage of silks with shoot bags, and re-inoculation after 1 week), and insertion of six Fusarium-colonized toothpicks into the silk channel. Only the injection through the husk leaves significantly increased fumonisin concentration and infection severity. Eller et al. (2008a) developed a methodology to identify resistance against F. verticillioides and identified superior genotypes. They preferred silk inoculation, which appears to be more important for the entrance of F. verticillioides. Furthermore, they compared four inoculation methods and found that the highest infection severity and largest genotypic differentiation were found when inoculum was inserted through the husks. Bush et al. (2004) compared five inoculation methods and concluded the most useful to be penetrating husks with pin bars and injecting inoculum down the silk channel.
Comparatively few data are available on the relationship between kernel and silk channel resistance. Lemmens (1999, 2010) found low correlation (r = 0.12), whereas Lo¨ ffler et al. (2010a) reported a much closer relationship (rP = 0.66) for a wider genetic stock. Chungu et al. (1996b) also found a correlation between the two traits (r = 0.77–0.89). Few genotypes exist with good resistance to both modes of fungal entry (Reid et al. 2003). The relationship between silk vs. kernel resistance is very important as two parallel methodologies would be cost-prohibitive in a breeding programme. Lo¨ ffler et al. 2010b has reported significant genotypic variances for kernel and silk channel resistance. The correlations between silk and kernel resistance were moderate (r = 0.66), but there were genotypes with very different resistance level with both methods. Therefore, authors suggest the use of both inoculation methods. There are arguments on the use of both inoculation techniques and on the number of isolates to be used, including whether to use a pure or mixed conidial suspension. The breeder is cautioned to carefully determine which mode of fungal entry is predominant at their research station and how heterogeneous is the Fusarium population at the station before making decisions on inoculation methods. In addition, the genotypic resistance that the breeder initially has to work with will influence this decision as sources of resistance to one mode of fungal entry may not be available in adapted material. For example, Presello et al. (2005) found kernel resistance for FER useful, but the sources for this resistance are very limited compared to sources for silk resistance. On the other hand, the breeders need the assistance of the research to answer better the questions they have.
Timing of inoculation
In a summary report, Jugenheimer (1976) cited Andrew (1954), who initiated GER by placing infected barley grains under the husk leaves 5–10 days after silking, that is, a very early form of silk channel inoculation, and further applied by spraying silks at silking to promote FER. In both cases, symptoms appeared after 1 month. This study can possibly be considered the first of many silk resistance tests. Since that time, many other researchers have performed silk and kernel inoculations at various stages of ear development. For GER, Papst et al. (2007) sprayed the silks 4–7 days after mid-silking and applied inoculum with wounding (toothpicks) 10–15 days after midsilking. For kernel resistance, later inoculation times result in a general decrease in disease severity for all species; though, it was not as strong as that reported with silk inoculations (Reid and Hamilton 1996, Reid et al. 2002). The best differentiation of genotypes was achieved when the kernel inoculation was performed 15 days after mid-silking (Reid and Hamilton 1996, Reid et al. 2002). Silva et al. (2007) used 14 days in regular tests; later inoculations (19, 21 and 23 days after mid-silking) resulted in significantly less disease severity. Reid et al. (1996a) used 2 ml of suspension inoculated 4–6 days after mid-silking for both GER and FER; earlier inoculations (2–3 days after mid-silking) led to very high infection severities, and later inoculations (10 days or more) resulted in little or no infection (Reid et al. 1992a, 2002). Schaafsma et al. (1997) concluded that the beginning of silk browning is the ideal time for the silk channel inoculation. They found irrigation useful for the initial stages of infection for both inoculation methods; this supports the data of Reid et al. (1996a). Silva et al. (2007) compared different inoculation methods. Robertson et al. (2006) and Eller et al. (2008a) used silk and direct ear inoculations at 10 days after mid-silking and 7 days later, respectively. Both methods gave replicable results for several superior lines. Disease levels between cultivars ranged from 20 to 50%. Interestingly, inoculation of the husk leaves resulted in significantly more severe infection of the grains.
Miller et al. (2007) found that silk-mediated F. graminearum infection reached the kernels in 7–9 days in susceptible genotypes, and 12–15 days in more resistant genotypes. These differences are of great importance in our goal of understanding disease severity and consequently resistance differences. This explains the roughly 1 week difference in timing of inoculation between the two inoculation methods. Adding 1 week to the 5–7 days postsilking of the silk channel inoculations, we achieve the optimum time for inoculating the kernels at the same stage in which the fungus growing down the silk would reach the kernels.
It should be mentioned that there are differences in the optimum timing of inoculation depending on the level of resistance. For early-maturing genotypes, the kernel development is more rapid than it is in later-maturing genotypes. Thus, a 10-day delay after mid-silking for kernel inoculations may be too late for some early genotypes. In large-scale breeding or screening programmes, the researcher should not rely just on chronological time for determining inoculation time; genotype maturity and growth stage must be taken into consideration as well as the effect of the environment in influencing growth and development of the ear. Control genotypes with known resistance levels in different maturity groups can be used to monitor this in large screenings such as a mapping population with very diverse silking and maturity rates.
Natural vs. Artificial Inoculation of Maize Ears
Disease severity after natural infection varies strongly from year to year, from location to location and with the length of the vegetation period; earlier hybrids normally show less ear rot infection and less toxin contamination (Papst et al. 2007). Late-sown or later hybrids tend to be more infected by F. graminearum and contaminated by DON than early ones (Manninger 1978, Blandino et al. 2009a). Good agronomy practices can also decrease the risk of fumonisin contamination (Blandino et al. 2009b). Weather conditions affect different Fusarium spp. in different ways (Reid et al. 1999), and the types and levels of physiological resistance strongly influence the severity observed. The many Fusarium spp. that are present in the fields cannot be controlled, and this complicates the comparison of natural and artificial inoculation results (Lo¨ ffler et al. 2010b). Ear tips that are fully covered by the husk leaves tend to be subject to more infection than those with early cob outgrowth as the humid period is longer in the former case (Butron et al. 2006). The optimum time for natural infection to occur is similar to that for artificial inoculation, about 10– 14 days after silking after which the infection severity sharply decreases. Natural infection is moderate to low in most years, and it is absent in some years and does not allow an efficient selection of genotypes with high resistance. These restrictions also explain the high ratio of susceptible inbreds and commercial hybrids bred under natural infection pressure. In 2010, an unusual strong epidemic caused by different FERs was observed in Hungary. Disease incidence vas very high, and severity was on average 20%. No genotype could be rated as resistant; rather, differences in susceptibility could be observed. In the most severely infected location, the incidence was between 28 and 87%, and the severity was between 8.5 and 29.4%. Most of the hybrids were susceptible or very susceptible, indicating the need for a more successful breeding against ear rots (Anonymous 2010).
Artificial inoculation methods have several advantages over relying on natural infection to create an outbreak of ear rot. The Fusarium spp. isolate, inoculation times and mode of entrance/infection pathway are known. The disease severity is normally much higher and results in more uniform ratings that make genotypic comparisons easier and reproducible especially when phenotyping for QTLs. Genotype differences are normally much larger for artificial inoculation regimes than for natural ones (Oberforster and Felder 2010). Silva et al. (2007) compared natural and artificial inoculation results and concluded that when only the husks were wounded, the sterile water control resulted in higher disease severity than the natural control. As the ranking order of the cultivars after wounding only and after inoculation did not seem to differ from that of the natural control, it was suggested to wounding only and to allow natural infection to proceed through the wounds in areas with high inoculum pressures as that found in the Andes where the study was conducted. Geographical areas such as these with reliable levels of natural infection are rare, especially in most maize-growing regions of the world.
In spite of all the concerns, both artificial and natural infections are of great significance. The genotypes selected under artificial infection pressure must demonstrate their superior resistance under natural infection pressures. For this reason, research that clarifies the relationships between artificial and natural infection results is of high priority. Lemmens (2010) observed a close correlation between the results of the silk channel method and natural infection (r = 0.75–0.96). In 2004, 2005 and 2006, Palavers?ic´ et al. (2010) found medium to close (r = 0.66, 0.61, 0.84) correlations between the silk channel and natural infection severity data. The data support the view that artificial and natural infection data tend to be closely correlated. This could mean that some form of complex resistance to different Fusarium spp. exists. The data suggest that this hypothesis may hold true.
Some maize breeders oppose the use of artificial inoculation methods. The reasons listed include the opinions that the natural infection severity is sufficient for efficient selection and that wounding at inoculation is far from the natural mode of infection and introduces instability in the system. However, in tests on commercial hybrids, these views cannot be fully justified as many hybrids from these programmes display considerable susceptibility. The effect of wounding seems to be overemphasized. In susceptible genotypes, the infection spreads rapidly to the unwounded, healthy kernels, whereas in resistant genotypes, the spread is very limited or does not occur at all. Some companies in many countries facing the food and feed safety problems have started intensive selection work with artificial inoculation methods.
Inheritance, Genotypic Differences and Sources of Resistance
Boling and Grogan (1965) estimated several additive, dominant and additive x dominant digenic epistatic gene effects. They estimated an average dominance of approximately 0.5, and the number of participating genes was estimated at 1.47, a relatively low number. Hart et al. (1984) reported that GER resistance is governed by genetic factors. Symptomatic and asymptomatic kernel infections have been studied in sweet corn hybrids between inbreds with different susceptibility levels (Nankam and Pataky 1996); a broad range of heritabilities for the two symptom groups were recorded, and resistance was determined to be controlled by several genes. In two maize populations, Robertson et al. (2006) found genotypic and phenotypic correlations between fumonisin and FER data of 0.96 and 0.40, and 0.86 and 0.64, respectively; heritability estimates for fumonisin were 0.75 and 0.86, and for ear rot resistance, 0.88 and 0.47, respectively. These high genetic correlations suggested that it is highly possible to reduce fumonisin contamination indirectly by increasing FER resistance levels. Using a silk channel inoculation method, Headrick and Pataky (1991) observed a significant maternal effect on hybrid performance. Eller et al. (2008a) concluded that the US hybrid maize crop was based on crosses between proprietary inbred lines, and many of them were developed from older, publicly developed inbreds representing a rather narrow gene pool. As the resistance level is seemingly unsatisfactory, they suggested a search for germplasms with higher resistance not closely related to this group.
Natural ear rot
There are many surveys for natural infection; in Europe, several Variety Offices regularly use the data for decisionmaking (Hertelendy et al. 2010, Oberforster and Felder 2010, Palavers?ic´ et al. 2010, Pastirc?a´k et al. 2010). In most years, the infection severities are low. In Hungary in 2010, the strongest epidemic was recorded for at least 20 years: the severity of maize ear rot across eight sites (the best-yielding hybrids were tested from many leading companies) was 35% with hybrid reactions ranging between 27 and 48%, the severity (coverage) by ear rot was 10.2% (range, 7–14%). The most infected site had an average disease incidence of 63% (range, 28–87%) and mean severity of 20% (range, 8–29%). This underlines two facts: (i) we cannot be satisfied with the results of breeding under natural infection regimes and (ii) a significant improvement in hybrid resistance is necessary. Similar outbreaks occur in other countries, and when this happens the result is high levels of localized disease incidence and severity.
Gibberella ear rot
The inheritance of resistance to Fusarium spp. is complex. Reid et al. (1992b) carried out a complete diallel analysis with 12 inbreds representing highly resistant to highly susceptible selected from a screening of 37 inbreds after silk channel inoculation. Both general (GCA) and specific combining ability (SCA) were significant. The GCA values were correlated to disease severity data; however, the performance of the hybrids could not be predicted based on the GCA of the parents. Four inbreds exhibited significant GCA for resistance to F. graminearum. Chungu et al. (1996a) tested the inheritance of kernel resistance by injecting a small amount of liquid inoculum into the centre of the ears. Generation mean analysis indicated that resistance to F. graminearum was under both simple (additive and dominance) and digenic (dominance x dominance) effects. Estimates of the number of factors affecting kernel resistance ranged from 4.6 to 13.7. Lemmens (1999) described a similar phenomenon – maize hybrids seem to possess different resistance levels as regards kernel and silk channel resistance. In some hybrids, Kova´ cs et al. (1994) found maternal effects, and in others, a paternal effect; kernel resistance to F. graminearum and F. culmorum in hybrids could be predicted only when both parents were solidly resistant.
Inbreds A632 and WP9 and their relatives exhibited GER resistance that was far above the average (Kova´ cs et al. 1994). Reid et al. (2000) observed resistance differences in sweet maize F. graminearum after silk channel inoculation, but the differences were relatively small and overall all genotypes were quite susceptible. In this study, DON levels increased rapidly such that by 2 weeks after silking concentrations were above 1 mg/kg. Reid et al. (2001a,b, 2003) have released eight maize inbreds (CO387, CO388, CO389, CO430, CO431, CO432, CO433, CO441) specifically bred for increased resistance to GER using both silk channel and kernel inoculation techniques. The latest release, CO441, has the highest published inbred resistance and possesses both silk and kernel resistance as well as acceptable grain yields when tested in combining ability trials (Reid et al. 2003). This it is feasible to develop resistant inbreds and high-yielding hybrids, thus breaking the resistance-yield barrier so often found when breeding for disease resistance. Inbreds developed with selection for GER also exhibited high levels of resistance to FER and common smut (Ustilago zeae) in inoculated trials (Reid et al. 2009), indicating that it may be possible to develop hybrids with resistance to multiple Fusarium spp. Schaafsma et al. (1997) tested 61 commercial hybrids for GER by silk channel and kernel inoculation methods and concluded that only two ranked highly resistant with both inoculation methods. de Oliveira et al. (2009) found some resistant sources among landraces with higher grain hardiness. Mesterha´ zy (1978, 1982, 1983) and Mesterha´ zy et al. (2000) tested maize hybrids against four isolates (two F. graminearum and two F. culmorum) with the toothpick method. The severity data on F. graminearum and F. culmorum correlated very closely, but the correlations with the less-pathogenic F. verticillioides and F. avenaceum were not convincing.
Research was initiated to find an indirect way for selecting to ear rot resistance. However, resistance to ear rot, stalk rot and seedling blight did not correlate, indicating that a preliminary selection at the seedling stage will not automatically result in higher ear or stalk rot resistance (Mesterha´ zy 1982). The data clearly showed (Mesterha´ zy and Kova´ cs 1988) that although the traits do not interrelate genetically, they may influence each other physiologically.
In most maize-growing regions, with the exception of developing countries, commercial varieties are hybrids; thus, it is critical that inbreds developed for improved GER carry this resistance into the hybrid. Kova´ cs et al. (1994) tested 18 hybrids with their respective inbred parents for GER. The mean for the maternal lines on scale 0–10 was 1.23, and that for the paternal lines was 2.40. Their calculated mean was 1.82, but the actual value was 1.08. This means that the hybrids had 41% less ear rot than the calculated mean. In six cases (33%), the hybrid was more resistant than the more resistant parental line. These data indicate that selection for resistant inbreds will result in resistant hybrids. Correlation between mother and hybrid performance was r g = 0.65 (P = 0.01), and between father and hybrid performance, r g= 0.85 (P = 0.001), indicating a stronger father influence. Recently, researchers in Germany conducted more formal studies to compare the relationship between inbred line resistance and toxin contamination (Bolduan et al. 2010, Lo¨ ffler et al. 2010b). It was concluded that effective line selection is possible, and toxin contamination follows closely the severity (rg = 0.88 between GER and FER, and rg = 0.77 for DON and FUM), indicating that the resistance to the different pathogens seems to be closely related. Toxin contamination is proportional to symptom severity when inoculation is performed with the same isolate. It seems that both proper line and testcross evaluations for ear rot resistance are equally important.
Some researchers have sought relations between seedling blight, ear rot and stalk rot (Mesterha´ zy and Kova´ cs 1988, Reid et al. 1996a), but failed to demonstrate any. Direct measurement of the resistance in the given organ is therefore necessary.
It is not clear what the mechanism of resistance to GER is in the resistant maize genotypes. Changes in silk flavone content and resistance to GER have been reported (Reid et al. 1992a,b). Assabgui et al. (1993) found a correlation of r = 0.70 between the (E)-ferulic acid content and resistance to F. graminearum. Bily et al. (2003) identified dehydrodimers of ferulic acid as a resistance component to F. graminearum ear rot. 4-Acetylbenzoxazolin-2-one (4-ABOA) was also reported to lead to a higher resistance level to GER (Miller et al. (1997). Recently, Cao et al. (2011) researched the role of hydoxycinnamic acids in resistance to GER and concluded that several changes in cell wall-bound compounds of silk tissues were observed after inoculation. Further studies are required in this research area.
Fusarium ear rot
Pascale et al. (2002) tested the resistance of 29 hybrids against F. verticillioides and F. proliferatum. The hybrid Mona was the most resistant. For fumonisin B1 + B2, Mona contained an average of 1.9 mg/kg for the 3 years, whereas a more susceptible hybrid Milpa had levels that reached 108 mg/kg. Fusarium proliferatum was more pathogenic, resulting in higher disease severities and smaller differences between the hybrids. Mona had a total fumonisin content of about 70 mg/ kg, while that of Milpa was about 50% less. Visibly infected grains had a high toxin concentration, indicating that separation of these grains from less-infected grain could significantly decrease the level of contamination. Presello et al. (2008) detected significant hybrid differences using silk channel inoculations with one isolate of F. verticillioides; the more susceptible hybrids also had higher natural infection levels. However, in symptomless grains, fumonisin could be measured in quantities exceeding the recommended limits. Diseased grains may be smaller, resulting yield losses up to 58% (Jovicevic and Sultan 1979, Warfield and Davis 1996) although F. verticillioides is thought to have no or only minimal impact on the yield. Sweet corn is generally considered to be a susceptible crop. Du Toit and Pataky (1999) observed highly significant resistance differences, but none of the hybrids was highly resistant. The silk channel injection method led to higher variation and was more laborious as compared with the toothpick method. Schjoth et al. (2008) found very good differences in resistance when medium disease pressure was applied, but these differences were less apparent when high pressure was applied. Clements et al. (2004) screened 1589 inbreds and a B73-type inbred for resistance to FER. On the basis of the fumonisin concentration, only 11 inbreds (A188, A682, B8, B66, C127, CK31, CM5, CQ201, H117, M14 and ND211) were superior and stable in the two trials conducted.
Pericarp thickness has been considered to play a role in FER resistance. The results of Ivic et al. (2008) clearly showed that no correlation between pericarp thickness and resistance exists in Croatian genotypes, so that breeding for this trait would not increase resistance. In contrast, Sampietro et al. (2009) identified various properties of the pericarp and its wax layer as resistance factors to F. verticillioides. These traits were consistent over 2 years under very differing ecological conditions. When the wax was removed, infection severity increased significantly. Waxy hybrids exhibit a higher average contamination of fumonisins (+440% in 2000 and +234% in 2001) than normal hybrids (Blandino and Reyneri 2007). Hoenisch and Davis (1994) observed a correlation between higher pericarp thickness and resistance. They considered that the thicker pericarp inhibits the fungus and may also act as a barrier to insect feeding. These studies may explain why sweet corn is so susceptible to both GER and FER; sweet corn varieties, a food crop, are intentionally bred to have thinner pericarps to improve texture upon eating. Long-chain alkanes on the surface of maize silks have also been implicated in resistance to GER (Miller et al. 2003).
The An2 gene encodes an ent-copalyl synthase gene that has a role in gibberellin synthesis. This gene was strongly upregulated after Fusarium infection of the silk (Harris et al. 2005). It was postulated that the gene might play a role in silk resistance. Haptoglobin-related protein (HRP) (Harris et al. 2005) genes have also been reported to play a potential role in resistance. Several flavonoids have been identified that have a possible role in resistance (Sekhon et al. 2006). Choi and Xu (2010) reported the cAMP signalizing pathway in F. verticillioides, which is important for conidiation and infection, may play a role in the infection process.
Farrar and Davis (1991) and Parsons and Munkvold (2010b) detected different thrips species on ears that increased the severity of FER infection. Husk looseness correlated with FER at the brown silk stage and also with the size of the thrips population. It was concluded that husk tightness plays an important part in epidemiology and disease development. Eller et al. (2008b) reported that the kernel moisture content influences the degree of ear rot. This concurs with studies on stalk rot causing pathological drydown and thus influencing GER (Mesterha´ zy 1983) as well as FER. Eller et al. (2008b) did not find a relation between ear rot resistance and yield performance. This would support the hypothesis that high resistance and high yield are not mutually exclusive for FER as well as GER.
Relationships between different ear rots
Many researchers work with more than one Fusarium spp. in their tests. Presello et al. (2004, 2006) found highly significant genotypic differences to different Fusarium spp. Resistance tests in both Canada and Argentina demonstrated correlations between F. graminearum and F. verticillioides resistance. Six populations (mostly being Andean landraces ARZM 01107, ARZM 07138, ARZM 10041, ARZM 13031, ARZM 16002 and Pora INTA) were identified that had very high levels of resistance to both pathogens and could be used as sources of high and stable resistance. Czembor and Ochodzki (2009) found higher ear rot resistance in flint genotypes than in dent ones. Lo¨ ffler et al. (2010a) reported the opposite experience. As the tested sets of genotypes were not the same in both studies, the data are not necessarily contradictory. Löffler et al. (2010a) observed good phenotypic and genotypic correlations in the flint and dent groups between F. graminearum and F. verticillioides. Negative correlations emerged between the silking date and ear rot severity for both Fusarium spp. (F. graminearum, rP = )0.28; F. verticillioides, rP = )0.26). So the lower severities for the later genotypes were confirmed. In the cited literature, there are several indications that resistance to F. graminearum and F. culmorum may be closely related (Mesterha´ zy 1982, 1983). As both cause GER, this is not surprising. For other Fusarium spp., however, the picture is not clear. In wheat, resistance protects all Fusarium spp. tested (Mesterha´ zy 2002, Mesterha´ zy et al. 2005). In maize, this remains to be clarified.
There is no clear evidence of resistance to specific toxins produced by the different Fusarium spp.; however, many studies (Reid et al. 1996a, Pascale et al. 1997, Perkowski et al. 1997, Reid and Sinha 1998) clearly indicate that severity of infection is highly correlated to toxin contamination, thus indicating a role of resistance in toxin regulation. Bolduan et al. (2009) found a high correlation between toxin contamination and the severity of the disease in response to F. graminearum with r = 0.94. Visual scoring can therefore be sufficient in selection work for GER resistance. For F. verticillioides, the infection severities are often significantly less and not as highly correlated to toxin levels (Miedaner et al. 2008); however, it was concluded that toxin analysis for both GER and FER are not necessary at all stages of breeding. Lo¨ ffler et al. (2011) found that the heritabilities for mycotoxin values were similar or higher than those found for ear rot data (both F. graminearum and F. verticillioides).
However, as the close correlation was not characteristic for all genotypes, they recommended separate testing of F. graminearum and F. verticillioides and corresponding mycotoxins. Henry et al. (2009) identified genotypes with good resistance to both F. verticillioides and Aspergillus flavus. Correlations between ear rot severities of the two pathogens (r = 0.72) and between aflatoxin and fumonisin concentrations (r = 0.61) led to the conclusion that good resistance to both species in the same genotype is attainable. Robertson-Hoyt et al. (2007b) came to the same conclusion.
It is interesting that the available literature does not concentrate on sources of resistance, the use of alien species for this purpose and the selection of inbred lines. Reid et al. (2009) identified inbreds with differing silk and kernel resistance types. In their study, silk channel resistance was investigated using one F. verticillioides and one F. graminearum isolate. It appeared that both additive and non-additive effects contributed to the resistance, and similarities in reaction to these pathogens were found. In tests in Szeged in Hungary, the correlation between the mean ear rot severity and DON contamination was r = 0.67, and for fumonisin B1-4, a total r = 0.68 was calculated, both significant at P = 0.1% (Toldi et al. 2008 and not published data). Presello et al. (2011) described resistance of inbreds selected from F2 populations and their hybrids inoculated with F. proliferatum. The populations were also inoculated by F. verticillioides and F. graminearum. Selection was similarly effective against F. graminearum and F. verticillioides.
Henry et al. (2009) tested 20 inbreds for F. verticillioides and A. flavus resistance. The ear rot values correlated for the two pathogens (r = 0.72, at P = 0.0002), as did the aflatoxin and fumonisin concentrations (r = 0.61, P = 0.0004), indicating that inbreds with aflatoxin resistance may be good sources for breeding for fumonisin resistance. Williams and Windham (2009) analysed fumonisin accumulation in a diallel analysis using A. flavus-resistant and A. flavus-susceptible inbreds inoculated with F. verticillioides and A. flavus. The inbreds Mp715 and MP 717 revealed high aflatoxin and fumonisin resistance; however, inbred Mp313E revealed resistance only against fumonisins, not aflatoxin. Farrar and Davis (1991) concluded that maize genotypes behaved very similarly to A. flavus and F. moniliforme (syn. F. verticillioides). Their data clearly show that resistance can be effective to different Fusarium spp., but also against other pathogens from different genera, for example A. flavus. There also appears to be a connection with common smut caused by Ustilago maydis (Reid et al. 2009). Eight GER-resistant inbreds were also resistant to Ustilago maydis. It should be further investigated whether this is caused by linkage or pleiotropy.
Farrar and Davis (1991) and Parsons and Munkvold (2010b) detected different thrips species on ears that increased the severity of FER infection. Husk looseness correlated with FER at the brown silk stage and also with the size of the thrips population. It was concluded that husk tightness plays an important part in epidemiology and disease development. Eller et al. (2008b) reported that the kernel moisture content influences the degree of ear rot. This concurs with studies on stalk rot causing pathological drydown and thus influencing GER (Mesterha´ zy 1983) as well as FER. Eller et al. (2008b) did not find a relation between ear rot resistance and yield performance. This would support the hypothesis that high resistance and high yield are not mutually exclusive for FER as well as GER.
Relationships between different ear rots
Many researchers work with more than one Fusarium spp. in their tests. Presello et al. (2004, 2006) found highly significant genotypic differences to different Fusarium spp. Resistance tests in both Canada and Argentina demonstrated correlations between F. graminearum and F. verticillioides resistance. Six populations (mostly being Andean landraces ARZM 01107, ARZM 07138, ARZM 10041, ARZM 13031, ARZM 16002 and Pora INTA) were identified that had very high levels of resistance to both pathogens and could be used as sources of high and stable resistance. Czembor and Ochodzki (2009)
found higher ear rot resistance in flint genotypes than in dent ones. Lo¨ ffler et al. (2010a) reported the opposite experience. As the tested sets of genotypes were not the same in both studies, the data are not necessarily contradictory. Lo¨ ffler et al. (2010a) observed good phenotypic and genotypic correlations in the flint and dent groups between F. graminearum and F. verticillioides. Negative correlations emerged between the silking date and ear rot severity for both Fusarium spp. (F. graminearum, rP = )0.28; F. verticillioides, rP = )0.26). So the lower severities for the later genotypes were confirmed. In the cited literature, there are several indications that resistance to F. graminearum and F. culmorum may be closely related (Mesterha´ zy 1982, 1983). As both cause GER, this is not surprising. For other Fusarium spp., however, the picture is not clear. In wheat, resistance protects all Fusarium spp. tested (Mesterha´ zy 2002, Mesterha´ zy et al. 2005). In maize, this remains to be clarified.
There is no clear evidence of resistance to specific toxins produced by the different Fusarium spp.; however, many studies (Reid et al. 1996a, Pascale et al. 1997, Perkowski et al. 1997, Reid and Sinha 1998) clearly indicate that severity of infection is highly correlated to toxin contamination, thus indicating a role of resistance in toxin regulation. Bolduan et al. (2009) found a high correlation between toxin contamination and the severity of the disease in response to F. graminearum with r = 0.94. Visual scoring can therefore be sufficient in selection work for GER resistance. For F. verticillioides, the infection severities are often significantly less and not as highly correlated to toxin levels (Miedaner et al. 2008); however, it was concluded that toxin analysis for both GER and FER are not necessary at all stages of breeding. Lo¨ ffler et al. (2011) found that the heritabilities for mycotoxin values were similar or higher than those found for ear rot data (both F. graminearum and F. verticillioides).
However, as the close correlation was not characteristic for all genotypes, they recommended separate testing of F. graminearum and F. verticillioides and corresponding mycotoxins. Henry et al. (2009) identified genotypes with good resistance to both F. verticillioides and Aspergillus flavus. Correlations between ear rot severities of the two pathogens (r = 0.72) and between aflatoxin and fumonisin concentrations (r = 0.61) led to the conclusion that good resistance to both species in the same genotype is attainable. Robertson-Hoyt et al. (2007b) came to the same conclusion.
It is interesting that the available literature does not concentrate on sources of resistance, the use of alien species for this purpose and the selection of inbred lines. Reid et al. (2009) identified inbreds with differing silk and kernel resistance types. In their study, silk channel resistance was investigated using one F. verticillioides and one F. graminearum isolate. It appeared that both additive and non-additive effects contributed to the resistance, and similarities in reaction to these pathogens were found. In tests in Szeged in Hungary, the correlation between the mean ear rot severity and DON contamination was r = 0.67, and for fumonisin B1-4, a total r = 0.68 was calculated, both significant at P = 0.1% (Toldi et al. 2008 and not published data). Presello et al. (2011) described resistance of inbreds selected from F2 populations and their hybrids inoculated with F. proliferatum. The populations were also inoculated by F. verticillioides and F. graminearum. Selection was similarly effective against F. graminearum and F. verticillioides.
Henry et al. (2009) tested 20 inbreds for F. verticillioides and A. flavus resistance. The ear rot values correlated for the two pathogens (r = 0.72, at P = 0.0002), as did the aflatoxin and fumonisin concentrations (r = 0.61, P = 0.0004), indicating that inbreds with aflatoxin resistance may be good sources for breeding for fumonisin resistance. Williams and Windham (2009) analysed fumonisin accumulation in a diallel analysis using A. flavus-resistant and A. flavus-susceptible inbreds inoculated with F. verticillioides and A. flavus. The inbreds Mp715 and MP 717 revealed high aflatoxin and fumonisin resistance; however, inbred Mp313E revealed resistance only against fumonisins, not aflatoxin. Farrar and Davis (1991) concluded that maize genotypes behaved very similarly to A. flavus and F. moniliforme (syn. F. verticillioides). Their data clearly show that resistance can be effective to different Fusarium spp., but also against other pathogens from different genera, for example A. flavus. There also appears to be a connection with common smut caused by Ustilago maydis (Reid et al. 2009). Eight GER-resistant inbreds were also resistant to Ustilago maydis. It should be further investigated whether this is caused by linkage or pleiotropy.
The lesson is clear. The literature indicates that toxin and ear rot severity data are correlated more often than not for FER and GER. Artificial inoculation followed by selection on the basis of disease severity is sufficient during the inbred selection process. Toxin evaluations should be made with initial parental selection for new populations, in the final stages of inbred development and in the second and third year of the official registration tests (in countries where this is applicable) to demonstrate the low toxin-producing capacity of the given hybrid. The close similarities in resistance to Fusarium, Gibberella and Aspergillus ear rots that have been described indicate with high probability that the resistance to these diseases may be common. However, exceptions have also been found. It is not known, however, whether the same resistance genes are responsible for this in a given maize genotype, or what is the interaction between the environment and different pathogen-specific genes. This will be an important research field in the future.
Molecular Genetics and QTL Mapping QTL mapping for GER resistance
In an F5 RIL population, Ali et al. (2005) found 11 QTLs for ear rot following silk inoculation and 18 QTLs after kernel inoculation (explaining 6–35% of the phenotypic variation). However, only two QTLs could be detected that were active across environments for silk resistance and only one for kernel resistance, indicating a strong influence of the environment. The majority of the favourable alleles came from the resistant parent CO387. Reinprecht et al. (2008) set out to identify the genes behind QTLs. About 100 genes were identified, among them chitinase and protein kinase were similar to previous gene-based markers that cosegregated with Fusarium resistance QTLs. Recently, Martin et al. (2011a,b) identified co-localized QTL for both GER resistance and reduced levels of DON in different mapping populations; they suggested that it may now be possible to conduct marker-assisted selection to improve GER resistance in the off-season but that classical phenotypic selection with field inoculations continue to be used during the cropping season.
QTL mapping for Fusarium ear rot resistance
Eller et al. (2008b) established that resistance to FER is determined by polygenes. Robertson-Hoyt et al. (2006c) tested two populations for resistance to F. verticillioides. In the FER population, seven QTLs were identified, explaining 47% of the phenotypic variation for FER, and nine were found for fumonisin content, explaining 67% of the variation. In the NCB population, five QTLs explained 31% of the FER variation and six QTLs with three epistatic interactions explained 81% of the phenotypic variation. Of the QTLs in the two populations, three QTLs for FER and two for fumonisin were mapped in similar positions. Two QTLs, localized on chromosome 4 and 5, appeared to be consistent in both populations. Ding et al. (2008) tested a RIL population of 187 genotypes for F. verticillioides resistance. Phenotyping was performed in four environments (location–year combinations). Two QTLs on chromosome 3 were identified with stability across environments. The major QTL explained 13– 22% of the phenotypic variation for FER. Perez-Brito et al. (2001) identified nine and seven QTLs in two populations, three of which were co-located. Kozhukhova et al. (2007) found a codominant marker RGA11 on the short arm of chromosome 1 for FER at 18.3 cM to the resistance locus in an F2 mapping population. For the SSR locus, the phi001 polymorphic amplicon 180 bp was identified. R2 or heritability values were not given.
Relationship of QTL to Fusarium ear rot resistance to other ear rots and agronomic traits
Robertson-Hoyt et al. (2007a) found that QTLs for F. verticillioides resistance were also effective against A. flavus. The genotypic correlations between ear rot data of the two pathogens (rG = 0.99) were very close. On chromosome 5, a large effect QTL was identified. The resistance QTLs against A. flavus and F. verticillioides were occasionally clustered on the same chromosomes. However, it was considered that a fine-scale genetic mapping will be necessary to distinguish linked QTLs, such as those in a resistance cluster from pleiotropic QTLs that influence resistance. This supports the view of common resistance to different Fusarium spp. (Robertson- Hoyt et al. 2007b). Robertson-Hoyt et al. (2007b) mapped two fumonisin QTLs to similar positions as that for grain yield, but the two QTLs were mapped to distinct genomic positions. Generally, close relations were not found between resistance and agronomic traits, and selection for higher resistance should therefore not unduly affect agronomic performance. A new attempt is the meta-analysis of QTLs associated with ear rot resistance (Xiang et al. 2010a,b). The data of 14 studies representing F. graminearum, F. verticillioides and A. flavus QTL studies were analysed; resistance QTLs against the three fungi were clustered on the same chromosomes. These data seem to support the idea of common resistance on QTL level. Of the 87 individual QTLs, 29 meta- QTLs were identified with 2–6 individual QTLs within a cluster. One resistance source can contribute to different clusters, for example CO387 influenced 18 of the 29 meta- QTLs. At present, it is not clear whether the QTLs in a cluster are individually effective to all three fungal pathogens or whether they are specialized to different fungal species and the cluster effect secures the broad sense resistance. We think based on wheat studies; the former is more likely (Mesterha´ zy et al. 2007).
Molecular genetics of Gibberella ear rot and mycotoxin accumulation
Yuan et al. (2008) found a guanylyl cyclase-like gene (Zmgc1) that ensures resistance to G. zeae; it is nearly identical to one resistance gene of the G. zeae-resistant line CO387. Jenczmionka and Schaefer (2005) described Gpmk1 MAP kinase disruption mutants and concluded that the infection process depends on the secretion of cell wall-degrading enzymes, especially during the early infection stages. Igawa et al. (2007) tested a ZEA-detoxifying enzyme in transgenic plants. It was expressed in the vegetation period and was also active up to 16 weeks during storage. The problem is that the disease was not or only moderately inhibited and other toxins may contaminate maize. Boutigny et al. (2008) surveyed naturally occurring mechanisms to the reduction of trichothecene toxins. They identified Class 1 mechanisms for detoxifying these toxins. Class 2 comprises mechanisms that result in reduced mycotoxin accumulation through inhibition of their biosynthesis. Some might work in practice, but their cost will certainly be considerable.
Molecular genetics of Fusarium ear rot and mycotoxin accumulation
Alexander et al. (2009) compared the biosyntheses of trichothecenes and fumonisins and concluded that the genes participating in these processes could possibly be used to enhance resistance to disease and reduce toxin contamination. Lanubile et al. (2010) found that in a resistant line, the assayed defencerelated genes (b-tubulin 2 and FUM21 genes of F. verticillioides) were transcribed at high levels before infection and provided basic defence against the fungus. In the susceptible line, the same genes are qualitatively induced from a basal level and respond specifically to pathogen infection. Zhang et al. (2011) identified the FvMK1 mitogen-activated protein kinase gene in F. verticillioides, which regulates conidiation, pathogenesis and also lowers the activity of the FUM1 and FUM8 genes.
Use of QTL mapping in practical breeding
The existence of meta-QTLs does not change the fact that most of the QTLs found are not validated and have only small effects (Robertson-Hoyt et al. 2006c, 2007a, Eller et al. 2008a,b). The 19 meta-QTLs (Xiang et al. 2010b) explain much of the resistance, but the individual QTLs are of small effect. Only one or two QTLs can be considered to have medium to large effects (Robertson-Hoyt et al. 2007a). Their additive (in some cases epistatic) effect seems to be proved. Therefore, their use for marker-assisted selection is limited at this time. Further complexity arises from the fact that in some hybrids, a maternal or paternal effect was dominant. The hybrid effect can be explained to some extent, but the resistance level in hybrids cannot be predicted with utmost certainty. This is similar to the situation in wheat (Buerstmayr et al. 2009). The results of Wilde et al. (2007) on wheat indicated that marker-assisted selection resulted in a twofold higher susceptibility in the progeny than in the phenotypically selected variant. We consider that a strong selection for increased resistance may give novel material for the development of new mapping populations that will allow the determination of new QTLs with higher effects, or identification of QTLs that ensure transgressive segregation. Other attempts with new genes may be of importance especially when the given enzymes can be identified in the QTL regions (Reinprecht et al. 2008).
Breeding Aspects
The development of genetic resistance to F. graminearum, F. verticillioides and other Fusarium spp. in maize should be a high priority in light of the toxins these species contaminate maize grain with (Reid et al. 1996a, Munkvold 2003b). Duvick (2001) suggested three theoretical approaches to decrease fumonisin contamination. Resistance is mentioned first, but resources may be limited. Molecular markers can also be applied to identify QTLs, but validated and effective markers are rare. A possibility in the future is to transfer resistance genes into maize and ensure higher resistance in this way, but at present no resistance genes are available. Presello et al. (2005) suggested pedigree selection to improve F. graminearum resistance. Both silk channel and kernel resistance were investigated: the selection for kernel resistance was more effective, and the resistance was more stable. Reid et al. (2001a,b, 2003) used modified pedigree selection to develop eight inbreds with improved GER resistance, some with high levels of both silk and kernel resistance. Researchers in Germany are using double haploid technology to develop GER-resistant inbreds (Martin et al. 2011b). Robertson-Hoyt et al. (2007a) did not find a close correlation between FER resistance and other traits such as yield; therefore, they hope that a strong selection for resistance will not result in lower yield and other unwanted consequences. This would question the yield penalty we mentioned regarding the high resistance in this paper. Reid et al. (2003) did develop an inbred, CO441, with high resistance to GER and excellent combining ability for yield. Mesterha´ zy et al. (2000) reported that resistant hybrids will be bred when both parents have good or excellent resistance; otherwise, the amount of resistance in the hybrid cannot be predicted with certainty. Ali et al. (2005) and Robertson et al. (2006) found transgressive segregation in maize; this could be utilized in maize breeding as was the case in wheat where the best F. graminearum-resistant source (Sumai-3) was bred from two medium-resistant lines.
Breeding for ear rot resistance involves two important steps. Munkvold (2003a) stresses the identification and use of native resistant sources. Breeding programmes could be based on these sources, because they are adapted and already available in the breeding nurseries. Nearly every paper cited reported significant differences in ear rot resistance; breeders need only start with the more resistant genotypes in their programme. de Oliveira et al. (2009) added that valuable breeding material can be identified among landraces; however, breeding with landraces can be time-consuming as many unwanted traits may have to be bred out of the populations first. Breeding can be started from hybrids made from crossing involving one or more lines with good resistance or from existing hybrids with proven superior resistance, providing no proprietary issues are involved that may restrict the use of the hybrids in a breeding programme. There is not much in the literature about when resistance selection should be performed in segregating generations. In most inbred breeding programmes, testing of the combining ability occurs in the S3, S4, or later generations; thus, the first resistance evaluation of testcrosses can be made at this time. However, as Lo¨ ffler et al. (2010a) has reported, many inbreds are susceptible as the infection pressure during inbreeding was not strong enough. Reid (1999) collected germplasms from around the world (adapted and unadapted) with moderate to high resistance to various ear pathogens, but no mention was made of how the inbreds were produced. However, as indicated in Reid et al. (2001a,b, 2003), inbreds were inoculated every generation in their development with the exception of few generation advances that were performed in off-season winter nurseries. Some of these inbreds resulted from crosses between a resistant inbred and an inbred with good agronomic performance, and other inbreds were selfed out of reciprocal recurrent selection populations that were subject to intense selection with artificial inoculations and toxin evaluations. Eller et al. (2010) also reported on inoculation during inbreeding; they concluded that the backcross method, that is normally used to transfer single major genes, was also successful in improving FER resistance. This might work for major QTLs, but for the normally polygenic trait it is less suitable as QTLs might be lost. In this case, the reciprocal recurrent selection suggested by Boling and Grogan (1965) might work better. Bolduan et al. (2010) suggested that the focus should be more on testing of hybrids and less on inbreds. We think both are important, as inbreds having superior resistance we can bred with to produce more resistant hybrids after several years. It is more important to start breeding of new resistant inbreds from materials with good or superior resistance. These sources with superior resistance should also be adapted to the target environment, otherwise commercially competitive inbreds are hard to achieve. If possible, both inbreds of a single cross should have good or excellent resistance to allow the breeding of commercial hybrids with maximum resistance.
Artificial inoculation methods must be extensively used in the testing of the new hybrids and their parents. Currently, most breeders use conidial suspensions, but toothpicks are also used by some. The interest in artificial inoculation is growing. It is interesting that fumonisin content has proven to be a more reliable trait than visual scores (Eller et al. 2008a). However, field evaluation of symptoms is rapid and can extend to thousands of genotypes, whereas any toxin analysis demands more time and is money-consuming. For both ear rots, the correlations between visual symptoms and toxin contamination are generally close (with a few exceptions). Accordingly, visual symptoms are suggested for screening. To verify the low toxin response of the new hybrids, at the end of the breeding process toxins should be evaluated (Bolduan et al. 2009).
Many breeders screen either for GER or for FER only, and few screen for both. Lo¨ ffler et al. (2010a) and Miedaner et al. (2008) stress the testing of inbreds and hybrids in several locations with a single isolate or a mixture of isolates. As no specialization is known, theoretically no difference exists between single isolates and mixtures of isolates of the same Fusarium spp. Therefore, the isolates may be used separately, and so (in the case of four isolates), the amount of resistance can be evaluated more precisely as with one single epidemic situation. Even so, resistance evaluation is made 2–3 years (Mesterha´ zy 1983, Kova´ cs et al. 1988). Some prefer isolate mixtures to avoid the possibility of a single isolate being less aggressive in a given field season, resulting in levels of disease too low to make accurate evaluations of resistance in 2–3 years (Reid et al. 1993). A mixture of isolates will decrease the environmental interaction. The resistance level will be the mean of those of several isolates used, so the extent of resistance can therefore be estimated better. A preselection of the isolates for aggressiveness is very important (Miedaner et al. 2010). As the aggressiveness varies (Mesterha´ zy 1985), the use of more isolates increases the reliability of the evaluations. As there are different ideas in these respects and solid scientific material is scare, further research is needed to give better suggestions for breeders.
Many studies have been published on the differing resistance mechanisms that may play a role in GER and FER resistance, and QTLs and genes have been found responsible for higher resistance. It is probable that some of these results will be used in breeding programmes. However, a well-working, methodical, phenotyping system must be used; otherwise, the gene expression cannot be checked at a practical level.
Regarding the two types of resistance, resistance to kernel infection is more stable and can be reproduced better than silk or silk channel resistance as the latter may be under more environmental influence and the window for fungal invasion is smaller as the fungus must grow down the silk channel before the silk dries out (Reid et al. 1992a, Reid and Hamilton 1996, Reid and Sinha 1998). Breeders must choose for themselves which mode of fungal entry is more important in their growing region. Some breeders separate their nurseries into silk channel and kernel resistance sections based on the sources of resistance used. Evaluation for both modes should be made during parental selection for new inbred development programmes and during the hybrid testing and evaluation of lines. In some cases where the major source of kernel infection is attributed to boring insects but these are controlled by Bt hybrids in commercial fields, it may be more practical to focus breeding efforts on silk channel resistance. However, it is important to remember that several reports indicate that the difference between these two modes may be less pronounced than normally anticipated.
Conclusions and Outlook
The maize–Fusarium system is complicated, but several conclusions can be drawn that are useful for resistance breeding programmes and resistance research. Breeding is a decisive part of the integrated production process from farm to fork (van der Fels-Klerx and Booij 2010). In all important maizegrowing regions, many Fusarium spp. occur at the same time, and their species structure depends on the region and the local weather conditions. Fusarium graminearum and F. verticillioides, the two most important species, and their close relatives have very diverging ecosystem requirements for their successful infection of a host plant. The available data indicate that common resistance may exist to both species in maize, consequently simplifying the breeding process. It is highly important that several authors have found correlations between the resistances to Aspergillus, Fusarium and Gibberella ear rots. This can lead to the hypothesis that resistance may be much wider than previously believed. This point is very important as hybrids are developed for global needs, and their resistance to all local Fusarium and other fungal populations should therefore be ensured whenever possible. Research on this complex resistance is needed to determine the cause of the high correlation between the natural and artificial inoculation results.
The data are clear in the respect that visual symptom severity and toxin contamination correlate closely for most species. Highly resistant genotypes demonstrate low disease severity and low toxin contamination. Specific toxin resistance is at present problematic. For example, in GER, DON may play virulence-increasing role, and glycosyl transferases (Lemmens et al. 2005) can also decrease the DON content in maize, but the significance of this for breeding is not yet known.
The registration process of new maize hybrids (in countries where this is applicable) should contain results from an artificial inoculation survey of the variety candidates. In this way, the susceptible and very susceptible hybrids could be banned from registration. This is the most effective way to improve food and feed safety worldwide. Unfortunately, as very few resistant hybrids currently exist, such a regulation would mean few if any hybrids get registered in some countries. With new regulations on allowable mycotoxin limits in several countries, breeding programmes to develop ear rot resistance are being initiated or strengthened with more resources. As a consequence, future hybrids may possess more resistance. Until this time comes, it is still paramount that farmers have access to ear rot rating data on hybrids that they may grow. This will allow farmers to choose hybrids with more resistance if ear rot is a significant threat on their farm. Countries need to organize an unbiased evaluation of all registered hybrids each year and make this information available to farmers until the time that enough resistant sources exist for resistant hybrids to be available.
The important fundamental steps of breeding for higher resistance have already been taken. These steps allow the breeding of inbreds and hybrids with a higher level of resistance than it was previously possible. However, many important questions are not yet answered. Additionally, more effective selection methods should be developed and existing methodology should be improved and standardized. The relationships between natural and artificial infection should also be clarified.
Food and feed safety requires more healthy grain (also silage); the investment in this sector of breeding and science may be expected to increase. We hope that this paper will contribute to the initiation of this process.
Acknowledgements
The authors express their thanks to the following projects: Hungarian Grant Agency NKTH: GAK (OMFB-01286/2004, OMFB 00313/ 2006), NAP-2-2007-0001 and the FP7 MYCORED KBBE-2007-2-5-05 and Deak Zrt Szeged, Hungary, Ontario Corn Producers Association, Ontario Pork, Agriculture and Agri-Food Canada and the Canadian Field Crop Research Alliance.
References
Afolabi, C. G., P. S. Ojiambo, E. J. A. Ekpo, A. Menkir, and R. Bandyopadhyay, 2008: Novel sources of resistance to fusarium stalk rot of maize in tropical Africa. Plant Dis., 92, 772—780.
Alexander, N. J., R. H. Proctor, and S. P. McCormick, 2009: Genes, gene clusters, and biosynthesis of trichothecenes and fumonisins in Fusarium. Toxin Rev. 28, 198—215.
Ali, M. L., J. H. Taylor, L. Jie, G. Sun, M. William, K. J. Kasha, L. M. Reid, and K. P. Pauls, 2005: Molecular mapping of QTLs for resistance to Gibberella ear rot, in corn, caused by Fusarium graminearum. Genome 48, 521—533.
Andrew, R. H., 1954: Disease of maize. Seventh FAO Hybrid Maize Meeting Report. FAO and UN.
Anonymous, 2005: Commission Regulation (EC) No 856/2005 of 6 June 2005: amending Regulation (EC) No 466/2001 as regards Fusarium toxins. Off. J. Eur. Union. 7.6.2005, L 143/3-8
Anonymous, 2006: Commission Recommendation of 17 August 2006 on the prevention and reduction of Fusarium toxins in cereals and cereal products (Text with EEA relevance). (2006/583/EC), 234/35- 40.
Anonymous, 2007: Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products. Off. J. Eur. Union 29.09.2007, L 255/14-17.
Anonymous, 2010: GOSZ-VSZT Kukorica Posztregisztra´ cio´ s Fajtaki´se´ rlet Ko´ rtani eredme´nyek, 2010 (Post registration variety test for maize by the Association of Cereal Producers and the National Seed Committee, Pathology results). Available at: http://www.vszt. hu/hu/gosz-vszt+kukorica+posztregisztr%C3%A1ci%C3%B3s+ k%C3%ADs%C3%A9rlet+2010+1.html (last accessed on May 27, 2011, 14:25:17).
Archer, T. L., C. Patrick, G. Schuster, G. Cronholm, E. D. Jr Bynum, and W. P. Morrison, 2001: Ear and shank damage by corn borers and corn earworms to four events of Bacillus thuringiensis transgenic maize. Crop Prot. 20, 139—144.
Assabgui, R. A., L. M. Reid, R. I. Hamilton, and J. T. Arnason, 1993: Correlation of kernel (e)-ferulic acid content of maize with resistance to Fusarium-graminearum. Phytopathology 83, 949—953.
Bacon, C. W., and D. M. Hinton, 1996: Symptomless endophytic colonization of maize by Fusarium moniliforme. Can. J. Bot. 74, 1195—1202.
Barrie` re, Y., O. Argillier, B. Michalet-Doreau, Y. He´bert, E. Guingo, C. Giauffret, and J. C. Mile, 1997: Relevant traits, genetic variation and breeding strategies in early silage maize. Agronomie 17, 395—411.
Barto´ k, T., A ´ . Sze´ csi, A. Szekeres, A ´ . Mesterha´ zy, and M. Barto´ k, 2006: Detection of new fumonisin mycotoxins and fumonisin-like compounds by reversed phase – high-performance liquid chromatography/ electrospray ionization – ion-trap mass spectrometry. Rapid Commun. Mass Spectrom. 20, 1—17.
Barto´ k, T., L. To¨ lgyesi, A. Szekeres, M. Varga, R. Bartha, A ´ . Sze´ csi, M. Barto´ k, and A ´ . Mesterha´ zy, 2010: Detection and characterization of twenty-eight isomers of fumonisin B1 (FB1) mycotoxin in a solid rice culture infected with Fusarium verticillioides by reserved phase high-performance liquid chromatography/electro spray ionization time-of-flight and ion trap mass spectrometry. Rapid Commun. Mass Spectrom. 24, 35—42.
Be´ke´ si, P., and K. Hinfner, 1970: Adatok a kukorica fuza´ riumos megbetegede´se´nek ismerete´hez (Data on the Fusarium spp. in maize). No¨ ve´nyve´delem 6, 13—18. (in Hungarian, English summary)
Berek, L., I. B. Perti, A ´ . Mesterha´ zy, J. Te´ren, and J. Molna´ r, 2001: Effect of mycotoxins on human immune functions in vitro. Toxicol. In Vitro 15, 25—30.
Bily, A. C., L. M. Reid, J. H. Taylor, D. Johnston, C. Malouin, A. J. Burt, B. Bakan, C. Regnault-Roger, K. P. Pauls, J. T. Arnason, and B. J. R. Philogene, 2003: Dehydrodimers of ferulic acid in maize grain pericarp and aleurone: resistance factors to Fusarium graminearum. Phytopathology 93, 712—719.
Bi´ro´ ne´ , G. M., 1975: Kukorica´n ka´ rosi´to´ Fusarium fajok elterjede´se e´ s toxikolo´ giai vizsga´ lata (Prevalence of Fusarium spp. in corn and their toxicity) (in Hungarian). PhD thesis, Go¨ do¨ ll}o. (Availability: Library of Hung. Acad. Sci., Budapest)
Bisby, G. R., and D. L. Bailey, 1923: Ear and root rots. Fourth Annual Report of the Survey of the Prevalence of Plant Diseases in the Dominion of Canada. 33 pp.
Blandino, M., and A. Reyneri, 2007: Comparison between normal and waxy maize hybrids for Fusarium-toxin contamination in NW Italy. Maydica 52, 127—134.
Blandino, M., A. Reyneri, and F. Vanara, 2009a: Effect of sowing time on toxigenic fungal infection and mycotoxin contamination of maize kernels. J. Phytopathol. 157, 7—14.
Blandino, M., A. Reyneri, G. Colombari, and A. Pietri, 2009b: Comparison of integrated field programmes for the reduction of fumonisin contamination in maize kernels. Field Crops Res. 111, 284—289.
Bolduan, C., T. Miedaner, W. Schipprack, B. S. Dhillon, and A. E. Melchinger, 2009: Genetic variation for resistance to ear rots and mycotoxins contamination in early European maize inbred lines. Crop Sci. 49, 2019—2028.
Bolduan, C., T. Miedaner, H. F. Utz, B. S. Dhillon, and A. E. Melchinger, 2010: Genetic variation in testcrosses and relationship between line per se and testcross performance for resistance to Gibberella ear rot in maize. Crop Sci. 50, 1691—1696.
Boling, M. B., and C. O. Grogan, 1965: Gene action affecting host resistance to Fusarium ear rot of maize. Crop Sci. 5, 305—307.
Boutigny, A. L., F. Richard-Forget, and C. Barreau, 2008: Natural mechanisms for cereal resistance to the accumulation of Fusarium trichothecenes. Eur. J. Plant Pathol. 121, 411—423.
Buerstmayr, H., T. Ban, and J. A. Anderson, 2009: QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: a review. Plant Breed. 128, 1—26.
Bush, B. J., L. M. Carson, M. A. Cubeta, W. M. Hagler, and G. A. Payne, 2004: Infection and fumonisin production by Fusarium verticillioides in developing maize kernels. Phytopathology 94, 88—93.
Butron, A., Santiago, R., P. Mansilla, C. Pintos-Varela, A. Ordas, and R. A. Malvar, 2006: Maize (Zea mays L.) genetic factors for preventing fumonisin contamination. J. Agric. Food. Chem. 54, 6113—6117.
de la Campa, R., Hooker, D. C., J. D. Miller, A. W. Schaafsma, and B. G. Hammond, 2005: Modeling effects of environment, insect damage, and Bt genotypes on fumonisin accumulation in maize in Argentina and the Philippines. Mycopathologia 159, 539—552.
Cao, A., L. M. Reid, A. Butro´ n, R. A. Malvar, X. C. Souto, and R. Santiago, 2011: Role of hydroxycinnamic acids in the infection of maize silks by Fusarium graminearum Schwabe. Mol. Plant Microbe Interact. 24, 1020—1026.
Choi, Y. E., and J. R. Xu, 2010: The cAMP signaling pathway in Fusarium verticillioides is important for conidiation, plant infection, and stress responses but not fumonisin production. Mol. Plant Microbe Interact. 23, 522—533.
Christensen, C. M. (ed.). 1982: Storage of Cereal Grains and their Products. American Association of Cereal Chemists, Inc., St. Paul, MN, 544 pp. ISBN: 0-913250-23-6.
Christensen, C. M., and H. H. Kaufmann, 1969: Grain Storage. The University of Minnesota Press, Minneapolis, 153 pp.
Christensen, J. J., and R. D. Wilcoxson, 1966: Stalk rot of corn. American Phytopathological Society, Monograph No.3. 59 pp.
Chungu, C., D. E. Mather, L. M. Reid, and R. I. Hamilton, 1996a: Inheritance of kernel resistance to Fusarium graminearum in maize. J. Hered. 87, 382—385.
Chungu, C., D. E. Mather, L. M. Reid, and R. I. Hamilton, 1996b: Comparison of techniques for inoculating maize silk, kernel and cob tissues with Fusarium graminearum. Plant Dis., 80, 81—84.
Clements, M. J., and D. G. White, 2004: Identifying sources of resistance to aflatoxin and fumonisin contamination in corn grain. J. Toxicol. Toxin Rev. 23, 381—396.
Clements, M. J., C. E. Kleinschmidt, C. M. Maragos, J. K. Pataky, and D. G. White, 2003: Evaluation of inoculation techniques for Fusarium ear rot and fumonisin contamination of corn. Plant Dis. 87, 147—153.
Clements, M. J., C. A. Maragos, J. K. Pataky, and D. G. White, 2004: Sources of resistance to fumonisin accumulation in grain and Fusarium ear and kernel rot of corn. Phytopathology 94, 251—260.
Czembor, E., and P. Ochodzki, 2009: Resistance of flint and dent maize forms for colonization by Fusarium spp. and mycotoxins contamination. Maydica 54, 263—267.
Ding, J. Q., X. M. Wang, S. Chander, J. B. Yan, and J. S. Li, 2008: QTL mapping of resistance to Fusarium ear rot using a RIL population in maize. Mol. Breed. 22, 395—403.
Dorn, B., H.-R. Forrer, S. Schu¨ rch, and S. Vogelsang, 2009: Fusarium species complex on maize in Switzerland: occurrence, prevalence, impact and mycotoxins in commercial hybrids under natural infection. Eur. J. Plant Pathol. 125, 51—61.
Dowd, P. F., 2001: Biotic and abiotic factors limiting efficacy of Bt corn in indirectly reducing mycotoxin levels in commercial fields. J. Econ. Entomol. 94, 1067—1074.
Du Toit, L. J., and J. K. Pataky, 1999: Reactions of processing sweet corn hybrids to Gibberella ear rot. Plant Dis. 83, 176—180.
Dufresne, M., T. van der Lee, S. B.MBarek, X. Xu, X. Zhang, T. Liu, C. Waalwijk, W. Zhang, G. H. J. Kema, and M.–. J. Daboussi, 2008: Transposon-tagging identifies novel pathogenicity genes in Fusarium graminearum. Fungal Genet. Biol. 45, 1552—1561.
Duncan, K. E., and R. J. Howard, 2010: Biology of maize kernel infection by Fusarium verticillioides. Mol. Plant Microbe Interact. 23, 6—16.
Duvick, J., 2001: Prospects for reducing fumonisin contamination of maize through genetic modification. Environ. Health Perspect. 109, 337—342.
Eller, M., J. Holland, and G. Payne, 2008a: Breeding for improved resistance to fumonisin contamination in maize. Toxin Rev. 27, 371—389.
Eller, M. S., L. A. Robertson-Hoyt, G. A. Payne, and J. B. Holland, 2008b: Grain yield and Fusarium ear rot of maize hybrids developed from lines with varying levels of resistance. Maydica 53, 231—237.
Eller, M. S., G. A. Payne, and J. B. Holland, 2010: Selection for reduced Fusarium ear rot and fumonisin content in advanced backcross maize lines and their topcross hybrids. Crop Sci. 50, 2249—2260.
Enerson, P. M., and R. B. Hunter, 1980: A technique for screening maize (Zea mays L.) for resistance to ear mould incited by Gibberella zeae (Schw.). Petch. Can. J. Plant Sci. 60, 1123—1128.
Farrar, J. J., and R. M. Davis, 1991: Relationship among ear morphology, western flower thrips, and Fusarium ear rot of corn. Phytopathology 81, 661—666.
van der Fels-Klerx, H. J., and C. H. J. Booij, 2010: Perspectives for geographically oriented management of Fusarium mycotoxins in the cereal supply chain. J. Food Prot. 73, 1153—1159.
Focke, I., 1966: Fusarium culmorum/W.G.Sm.) und F. moniliforme Sheld. als Erreger von Kolbenfaulen des Maises (Zea mays L.) im mitteldeutschem Raum. Wissenschaftliche Zeitschrift der Wilhelm- Pieck-Universita¨ t Rostock. Naturwissenschaftliche Reihe. Wilhelm- Pieck-Universita¨ t Rostock, Rostock. Wiss. Z. Univ. Rostock, Nat. Wiss. Reihe 15, 219—227.
Focke, I., and W. Ku¨ hnel, 1964: Die Weissfaule der Maiskolben (Fusarium poae PK/Wr.). Nachrichtenblatt fu¨ r Deutschen Pflanzenschutzdienst (Berlin) 18, 1—8.
Folcher, L., M. Jarry, A. Weissenberger, F. Gerault, N. Eychenne, M. Delos, M. Delos, and C. Regnault-Roger, 2009: Comparative activity of agrochemical treatments on mycotoxin levels with regard to corn borers and Fusarium mycoflora in maize (Zea mays L.) fields. Crop Prot. 28, 302—308.
Foley, D. C., 1959: Systemic infection of corn by Fusarium moniliforme. Phytopathology 49, 538.
Garcia, D., Ramos, A. J., V. Sanchis, and S. Mari´n, 2009: Predicting mycotoxins in foods: a review. Food Microbiol. 26, 757—769.
Garcia-Aguirre, G., and R. Martinez-Flores, 2010: Fusarium species from corn kernels recently harvested and shelled in the fields in the Ciudad Serdan Region, Puebla. Rev. Mex. Biodivers. 81, 15—20.
Gelderblom, W. C. A., K. Jaskiewicz, W. F. O. Marasas, P. G. Thiel, R. M. Horak, R. Vleggaar, and N. P. J. Kriek, 1988: Fumonisins – novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl. Environ. Microbiol. 54, 1806—1811.
Gergely, L., P. Hertelendy, Z. Birta-Vas, Sz. Szla´ vik, L. La´ za´ r, and J. Csapo´ , 2010: The evaluation of the diseases caused by Fusarium fungi at the Central Agricultural Office. In: A ´ . Mesterha´ zy (ed.), Workshop for Variety Registration in Cereals for Fusarium Resistance, 27—28. March 23–24, 2010, Szeged, Hungary.
Goertz, A. 2009: Auftreten der Fusarium-Kolbenfa¨ ule im Maisanbau in Deutschland und Maßnahmen zur Vermeidung der Mykotoxinbelastung in Maisko¨ rnern. Inaugural Dissertation, University of Bonn, 138 pp.
Goertz, A., E.-C. Oerke, U. Steiner, C. Waalwijk, I. Vries, and H.-W. Dehne, 2008: Biodiversity of Fusarium species causing ear rot of maize in Germany. Cereal Res. Commun. 36, 617—622.
Goertz, A., S. Zuehlke, M. Spiteller, U. Steiner, H. W. Dehne, C. Waalwijk, I. de Vries, and E. C. Oerke, 2010: Fusarium species and mycotoxin profiles on commercial maize hybrids in Germany. Eur. J. Plant Pathol. 128, 101—111.
Harris, L. J., A. Saparno, A. Johnston, S. Prisic, M. Xu, S. Allard, A. Kathiresan, T. Ouellet, and R. J. Peters, 2005: The maize An2 gene is induced by Fusarium attack and encodes an ent-copalyl diphosphate synthase. Plant Mol. Biol. 59, 881—894.
Harrison, L. R., B. N. Colvin, J. T. Greene, L. E. Newman, and R. J. Cole, 1990: Pulmonary edema and hydrothorax in swine produced by fumonisin B1, a toxic metabolite of Fusarium moniliforme. J. Vet. Diagn. Invest. 2, 217—221.
Hart, L. P., E. Gendloff, and E. C. Rossman, 1984: Effect of corn genotypes on ear rot infection by Gibberella zeae. Plant Dis. 68, 296—298.
Headrick, J. M., and J. K. Pataky, 1991: Maternal influence on the resistance of sweet corn lines to kernel infection by Fusarium moniliforme. Phytopathology 81, 268—274.
Henry, W. B., W. P. Williams, G. L. Windham, and L. K. Hawkins, 2009: Evaluation of maize inbred lines for resistance to Aspergillus and Fusarium ear rot and mycotoxin accumulation. Agron. J. 101, 1219—1226.
Hertelendy, P., Z. Birta-Vas, and S. Szla´ vik, 2010: Evaluation methods of Fusarium stalk rot and Fusarium ear rot for the registration process in Hungary. In: A ´ . Mesterha´ zy (ed.), Workshop for Variety Registration in Cereals for Fusarium Resistance, 21—22. March 23– 24, 2010, Szeged, Hungary.
Hoenisch, R. W., and R. M. Davis, 1994: Relationship between kernel pericarp thickness and susceptibility to Fusarium ear rot in field corn. Plant Dis. 78, 517—519.
Igawa, T., N. Takahashi-Ando, N. Ochiai, S. Ohsato, T. Shimizu, T. Kudo, I. Yamaguchi, and M. Kimura, 2007: Reduced contamination by the Fusarium mycotoxin zearalenone in maize kernels through genetic modification with a detoxification gene. Appl. Environ. Microbiol. 73, 1622—1629.
Iglesias, J., D. A. Presello, G. Botta, G. A. Lori, and C. M. Fauguel, 2010: Aggressiveness of Fusarium Section Liseola isolates causing maize ear rot in Argentina. J. Plant Pathol. 92, 205—211.
International Agency for Research on Cancer (IARC), 1993: Monograph 56: Some Naturally Occurring Substances: Some Food Items and Constituents Heterocyclic Amines and Mycotoxins. IARC, Lyon, France.
Ivic,D.,M.Cabric,B.Palaversic,andB.Cvjetkovic, 2008:Nocorrelation between pericarp thickness and Fusarium ear rot (Fusarium verticillioides) in Croatian maize hybrids and lines. Maydica 53, 297—301.
Jenczmionka, N. J., and C´ . W. Schaefer, 2005: The Gpmk1 MAP kinase of Fusarium graminearum regulates the induction of specific secreted enzymes. Curr. Genet. 47, 29—36.
Jovicevic, B., and M. Sultan, 1979: Effect of Fusarium ear rot on corn yields. Zastita Bilja 30, 223—228.
Jugenheimer, R. W., 1976: Corn Improvement, Seed Production and Uses. John Wiley and Sons, New York, London, Sydney, Toronto, 670 pp.
Kellerman, T. S., W. F. O. Marasas, P. G. Thiel, W. C. A. Gelderblom, M. Cawood, and J. A. W. Coetzer, 1990: Leukoencephalomalacia in two horses induced by oral dosing of fumonisin B1. Onderstepoort J. Vet. Res. 57, 269—275.
Koehler, B., 1942: Natural mode of entrance of fungi into corn ears and some symptoms that indicate infection. J. Agric. Res. 64, 421—442.
Koehler, B., 1950: Corn stalk rot and ear studies. Proceeding of 5th Corn Research Conference. 33—45. American Seed Trade Association.
Koehler, B., 1957: Pericarp injuries in seed corn. Prevalence in dent corn and relation to seedling blights. Univ. Ill. Agric. Exp. Sta. Bulletin 617, 72.
Koehler, B., 1959: Corn ear rots in Illinois. Ill. Agric. Exp. Sta. Bulletin 639, 87.
Kova´ cs, G., Jr, G.-ne´ . Kova´ cs, and A ´ . Mesterha´ zy, 1988: Kukoricahibridek cs}o- e´s sza´ rfuza´riummal szembeni ellena´ llo´ sa´ ga e´ s mechanikai szila´ rdsa´ ga. (Resistance of corn hybrids to fusarial stalk rot and ear rot and their mechanical stalk characteristics). No¨ ve´nytermele´s 37, 1—12.
Kova´ cs, K., G. Jr Kova´ cs, and A ´ . Mesterha´ zy, 1994: Expression of resistance to fusarial ear blight in corn inbreds and their hybrids. Maydica 39, 187—190.
Kozhukhova, N. E., M. Syvolap Yu, B. F. Varenyk, and V. M. Sokolov, 2007: Marking of the loci encoding maize resistance to Fusarium. Cytol. Genet. 41, 37—41.
Lanubile, A., P. Luca, and M. Adriano, 2010: Differential gene expression in kernels and silks of maize lines with contrasting levels of ear rot resistance after Fusarium verticillioides infection. J. Plant Physiol. 167, 1398—1406.
Lemmens, M., 1999: Anwendung ku¨ nstlicher Inokulationsmethoden zur Kolbenfusariose-Resistenzbestimmung bei Mais. 50. Tagung der Vereinigung O¨ sterreichischer Pflanzenzu¨ chter, BAL Gumpenstein, 63—68.
Lemmens, M., 2010: Fusarium ear rot resistance testing in maize: natural infection versus artificial inoculation. In: A ´ . Mesterha´ zy (ed.), Workshop for Variety Registration in Cereals for Fusarium Resistance, 18—20. March 23–24, 2010, Szeged, Hungary.
Lemmens, M., U. Scholz, F. Berthiller, C. DallAsta, A. Koutnik, R. Schuhmacher, G. Adam, H. Buerstmayr, A ´ . Mesterhazy, R. Krska, and P. Ruckenbauer, 2005: The ability to detoxify the mycotoxin deoxynivalenol co-localizes with a major QTL for Fusarium head blight resistance in wheat. Mol. Plant Microbe Interact. 18, 1318—1324.
Lo¨ ffler, M., B. Kessel, M. Ouzunova, and T. Miedaner, 2010a: Population parameters for resistance to Fusarium graminearum and Fusarium verticillioides ear rot among large sets of early, mid-late and late maturing European maize (Zea mays L.) inbred lines. Theor. Appl. Genet. 120, 1053—1062.
Lo¨ ffler, M., T. Miedaner, B. Kessel, and M. Ouzunova, 2010b: Mycotoxin accumulation and corresponding ear rot rating in three maturity groups of European maize inoculated by two Fusarium species. Euphytica 174, 153—164.
Lo¨ ffler, M., B. Kessel, M. Ouzunova, and T. Miedaner, 2011: Covariation between line and testcross performance for reduced mycotoxin concentrations in European maize after silk channel inoculation of two Fusarium species. Theor. Appl. Genet. 121, 925—934.
Logrieco, A., G. Mule, A. Moretti, and A. Bottalico, 2002: Toxigenic Fusarium species and mycotoxins associated with maize ear rot in Europe. Eur. J. Plant Pathol. 108, 597—609.
Manninger, I., 1978: Evaluation of corn diseases in breeding for resistance and the qualification of wheat varieties (in Hungarian with English captions and abstract). No¨ ve´nytermele´ s 27, 7—10.
Mari´n, S., I. Hodzic, A. J. Ramos, and V. Sanchis, 2008: Predicting the growth/nogrowth boundary and ochratoxin A production by Aspergillus carbonarius in pistachio nuts. Food Microbiol. 25, 683—689.
Martens, J. W., W. L. Seaman, and T. G. Atkinson, 1984: Diseases of Field Crops in Canada. An Illustrated Compendium. Canadian Phytopathological Society, Harrow, Ontario, Canada.
Martin, M., T. Miedaner, B. S. Dhillon, U. Ufermann, B. Kessel, M. Ouzunova, W. Schipprack, and A. E. Melchinger, 2011a: Colocalization of QTL for Gibberella ear rot resistance and low mycotoxin contamination in early European maize. Crop Sci. 51, 1935—1945.
Martin, M., D. D. Miedaner, B. Schwegler, M. Kessel, B. S. Ouzunova, W. Dhillon, and H. F. Schipprack, 2011b: Comparative QTL mapping for Gibberella ear rot resistance and reduced deoxynivalenol contamination across connected maize populations. Crop Sci. 57, 1935—1945.
Menkir, A., R. L. Brown, R. Bandyopadhyay, Z.-Y. Chen, and T. Cleveland, 2006: A USA-Africa collaborative strategy for identifying, characterizing, and developing maize germplasm with resistance to aflatoxin contamination. Mycopathologia 162, 225—232.
Mesterha´ zy, A ´ ., 1978: Kukoricahibridek cs}o- e´ s sza´ rfuza´riummal szembeni ellena´ llo´ sa´ ga e´s mechanikai szila´ rdsa´ ga (Resistance of corn hybrids to stalk rot and ear rot and the mechanical strength of the stalk). No¨ ve´nytermele´s 37, 1—11.
Mesterha´ zy, A ´ ., 1979: Stalk splitting as a method for evaluating stalk rot of corn. Plant Dis. Reporter 63, 227—231.
Mesterha´ zy, A ´ ., 1982: Resistance of corn to Fusarium ear rot and its relation to seedling resistance. Phytopathologische Zeitschrift 103, 218—231.
Mesterha´ zy, A ´ ., 1983: Relationship between resistance to stalk rot and ear rot of corn influenced by rind resistance, premature death and the rate of drying of the ear. Maydica 28, 425—437.
Mesterha´ zy, A ´ ., 1985: Effect of seed production area on the seedling resistance of wheat to Fusarium seedling blight. Agronomie (Paris), 5, 491—497.
Mesterha´ zy, A ´ ., 1995: Types and components of resistance against Fusarium head blight of wheat. Plant Breed. 114, 377—386.
Mesterha´ zy, A ´ ., 2002: Role of deoxynivalenol in aggressiveness of Fusarium graminearum and F. culmorum and in resistance to Fusarium head blight. Eur. J. Plant Pathol. 108, 675—684.
Mesterha´ zy, A ´ ., and K. Kova´ cs, 1988: Breeding corn against fusarial stalk rot, ear rot and seedling blight. Acta Phytopathol. Acad. Sci. Hung. 21, 231—249.
Mesterha´ zy, A ´ ., and M. Vojtovics, 1977: A kukorica Fusarium okozta ferto˜ zo¨ ttse´ge´nek vizsga´ lata 1972-1975-ben. (Investigation of Fusarium infection in corn 1972–1975). No¨ ve´nytermele´ s 26, 367—378.
Mesterha´ zy, A ´ ., T. Barto´ k, C. M. Mirocha, and R. Komoro´ czy, 1999: Nature of resistance of wheat to Fusarium head blight and deoxynivalenol contamination and their consequences for breeding. Plant Breed. 118, 97—110.
Mesterha´ zy, A ´ ., G. Jr Kova´ cs, and K. Kova´ cs, 2000: Breeding resistance for Fusarium ear rot (FER) in corn. Genetika 32, 495—505.
Mesterha´ zy, A ´ ., T. Barto´ k, G. Ka´ szonyi, M. Varga, B. To´ th, and J. Varga, 2005: Common resistance to different Fusarium spp. causing Fusarium head blight in wheat. Eur. J. Plant Pathol. 112, 267—281.
Mesterha´ zy, A ´ ., H. Buerstmayr, B. To´ th, Sz. Lehoczki-Krsjak, A ´ . Szabo´ -Heve´ r, and M. Lemmens, 2007: An improved strategy for breeding FHB resistant wheat must include Type I resistance. In: R. Clear (ed.), Proceedings of the 5th Canadian Workshop on Fusarium Head Blight, 51—66. November 27–30, 2007, Delta Winnipeg.
Miedaner, T., M. Lo¨ ffler, C. Bolduan, B. Kessel, M. Ouzunova, V. Mirdita, and A. E. Melchinger, 2008: Genetic variation for resistance and mycotoxin content of European maize inoculated with Fusarium graminearum and F. verticillioides. Cereal Res. Commun. 36(Suppl B), 45—48.
Miedaner, T., C. Bolduan, and A. E. Melchinger, 2010: Aggressiveness and mycotoxin production of eight isolates each of Fusarium graminearum and Fusarium verticillioides for ear rot on susceptible and resistant early maize inbred lines. Eur. J. Plant Pathol. 127, 113—123.
Miller, J. D., M. Miles, and D. A. Fielder, 1997: Kernel concentrations of 4-acetylbenzoxazolin-2-one and diferuloylputrescine in maize genotypes and Gibberella ear rot. J. Agric. Food Chem. 45, 4456—4459.
Miller, S. S., L. M. Reid, G. Butler, S. P. Winter, and N. J. McGoldrick, 2003: Long chain alkanes in silk extracts of maize genotypes with varying resistance to Fusarium graminearum. J. Agric. Food. Chem. 51, 6702—6708.
Miller, S. S., L. M. Reid, and L. J. Harris, 2007: Colonization of maize silks by Fusarium graminearum, the causative organism of Gibberella ear rot. Can. J. Bot. 85, 369—376.
Mirocha, C. J., 1974: Isolation, detection, and quantization of zearalenone in maize and barley. J. Assoc. Off. Anal. Chem. 57, 1104—1974.
Munkvold, G. P., 2003a: Epidemiology of Fusarium diseases and their mycotoxins in maize ears. Eur. J. Plant Pathol. 109, 705—713.
Munkvold, G. P., 2003b: Cultural and genetic approaches to managing mycotoxins in maize. Annu. Rev. Phytopathol. 41, 99—116.
Munkvold, G. P., R. L. Hellmich, and W. B. Showers, 1997a: Reduced Fusarium ear rot and symptomless infection in kernels of maize genetically engineered for European corn borer resistance. Phytopathology 87, 1071—1077.
Munkvold, G. P., D. C. McGee, and W. M. Carleton, 1997b: Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology 87, 209—217.
Murillo-Williams, A., and G. P. Munkvold, 2008: Systemic infection by Fusarium verticilliodes in maize plants grown under three temperature regimes. Plant Dis. 92, 1695—1700.
Nagy, E., and I. Cabulea, 1996: Breeding maize for tolerance to Fusarium stalk rot and ear rot stress. Romanian Agr. Res. – INCDA Fundulea. 5–6, 43—58.
Nankam, C., and J. K. Pataky, 1996: Resistance to kernel infection by Fusarium moniliforme in the sweet corn inbred IL125b. Plant Dis. 80, 593—598.
Nelson, P. E., T. A. Tousson, and W. F. O. Marasas, 1983: Fusarium species. An illustrated manual for identification. The Pennsylvania State University Press, University Park and London, 193 pp.
Nicolaisen, M., S. Suproniene, L. K. Nielsen, I. Lazzaro, N. H. Spliid, and A. F. Justesen, 2009: Real-time PCR for quantification of eleven individual Fusarium species in cereals. J. Microbiol. Meth., 76, 234—240.
Oberforster, M., and H. Felder, 2010: Systems for evaluating the susceptibility of cereal and maize cultivars to Fusarium in Austria. In: A ´ . Mesterha´ zy (ed.), Workshop for Variety Registration in Cereals for Fusarium Resistance, 31—33. March 23–24 2010, Szeged, Hungary.
de Oliveira, T. R., D. D. Jaccoud, L. Henneberg, M. D. Michel, I. M. Demiate, A. T. B. Pinto, M. Machinski, and A. C. Barana, 2009: Maize (Zea mays L.) landraces from the Southern Region of Brazil: contamination by Fusarium spp., zearalenone, physical and mechanical characteristics of the kernels. Braz. Arch. Biol. Technol. 52, 11—16.
Palavers?ic´ , B., M. Jukic´ , I. Buhinic?ek, A. Vragolovic´ , and Z. Kozic´ , 2010: Several years of testing maize hybrids for resistance to Gibberella ear rot. In: A ´ . Mesterha´ zy (ed.), Workshop for Variety Registration in Cereals for Fusarium Resistance, 20—21. March 23– 24, 2010, Szeged, Hungary.
Pappelis, A. J. 1984: The maize root rot, stalk rot. In: Sorghum Root and Stalk Rots, A Critical Review. Proceedings of the Consultative Group Discussion on Research Needs and Strategies for Control of Sorghum Root and Stalk Rot Diseases, 155—172. 27 November–2 December 1983, ICRISAT, Bellagio, Italy.
Papst, C., H. F. Utz, A. E. Melchinger, J. Eder, T. Magg, D. Klein, and M. Bohn, 2005: Mycotoxins produced by Fusarium spp. in isogenic Bt vs. non-Bt maize hybrids under European corn borer pressure. Agron. J. 97, 219—224.
Papst, C., J. Zellner, S. Venkataratnam, and J. Eder, 2007: Fusarium – Problematik bei Ko¨ rnermais (Zea mais L.). Gesunde Pflanzen 59, 7—16.
Parsons, M. W., and G. P. Munkvold, 2010a: Associations of planting date, drought stress, and insects with Fusarium ear rot and fumonisin B1 contamination in California maize. Food Addit. Contam. Part A 27, 591—607.
Parsons, M. W., and G. P. Munkvold, 2010b: Relationships of immature and adult thrips with silk-cut, Fusarium ear rot and fumonisin B-1 contamination of maize in California and Hawaii. Plant. Pathol. 59, 1099—1106.
Pascale, M., A. Visconti, M. Pronczuk, H. Wisniewska, and J. Chelkowski, 1997: Accumulation of fumonisins in maize hybrids inoculated under field conditions with Fusarium moniliforme Sheldon. J. Sci. Food Agric. 74, 1—6.
Pascale, M., A. Visconti, and J. Chelkowski, 2002: Ear rot susceptibility and mycotoxin contamination of maize hybrids inoculated with Fusarium species under field conditions. Eur. J. Plant Pathol. 108, 645—651.
Pastirc?a´ k, M., M. Lemmens, and A. S? roba´ rova´ , 2010: Resistance of maize hybrids to ear rot. In: A ´ . Mesterha´ zy (ed.), Workshop for Variety Registration in Cereals for Fusarium Resistance, 34—35. March 23–24, 2010, Szeged, Hungary.
Pauls, P., J. Taylor, G. Sun, M. William, J. Liu, K. Kasha, and L. M. Reid, 2001: Identification of molecular markers for resistance to Fusarium graminearum in maize. Canadian Workshop on Fusarium Head Blight, 4—6. Ottawa.
Perez-Brito, D., D. Jeffers, D. Gonzalez-de-Leon, M. Khairallah, and M. Cortes-Cruz, 2001: QTL mapping of Fusarium moniliforme earrot resistance in highland maize, Mexico. Agrosciencia 35, 181—196.
Perkowski, J., M. Pronczuk, and J. Chelkowski, 1997: Deoxynivalenol and acetyldeoxyniva-lenol accumulation in field maize inoculated by F. graminearum. J. Phytopathol. 145, 113—116.
Pestka, J. J., and G. S. Bondy, 1994: Immunotoxic effects of mycotoxins. In: J. D. Miller, and H. L. Trenholm (eds), Mycotoxins in Grain: Compounds other than Aflatoxin, 339—358. The American Phytopathological Society, St. Paul, MN.
Plienegger, J., and M. Lemmens, 2002: Kolbenfa¨ ule – Gibt es Sortenunterschiede? Mais 30, 95—97.
Prelusky, D. B., B. A. Rotter, and R. G. Rotter, 1994: Toxicology of mycotoxins. In: J. D. Miller, and H. L. Trenholm (eds), Mycotoxins in Grain: Compounds other than Aflatoxin, 359—403. The American Phytopathological Society, St. Paul, MN.
Presello, D. A., L. M. Reid, and D. E. Mather, 2004: Resistance of Argentine maize germplasm to Gibberella and Fusarium ear rots. Maydica 49, 73—81.
Presello, D. A., L. M. Reid, G. Butler, and D. E. Mather, 2005: Pedigree selection for Gibberella ear rot resistance in maize populations. Euphytica 143, 1—8.
Presello, D. A., J. Iglesias, G. Botta, L. M. Reid, G. A. Lori, and G. H. Eyherabide, 2006: Stability of maize resistance to the ear rots caused by Fusarium graminearum and F. verticillioides in Argentinean and Canadian environments. Euphytica 147, 403—407.
Presello, D. A., G. Botta, J. Iglesias, and G. H. Eyherabide, 2008: Effect of disease severity on yield and grain fumonisin concentration of maize hybrids inoculated with Fusarium verticillioides. Crop Prot. 27, 572—576.
Presello, D. A., A. O., Pereyra., J. Iglesias, C. M. Fauguel, D. A. Sampietro, and G. H. Eyhe´rabide, 2011: Responses to selection of S5 inbreds for broad-based resistance to ear rots and grain mycotoxin contamination caused by Fusarium spp. in maize. Euphytica, 178, 23—29.
Reid, L. M. 1999: Breeding for resistance to ear rot in corn. Canadian Workshop on Fusarium Head Blight (Colloque Canadien Sur La Fusariose), 66–68. November 28–30, 1999, Holiday Inn Crown Plaza Winnipeg, Manitoba.
Reid, L. M., and R. I. Hamilton, 1996: Effects of inoculation position, timing, macroconidial concentration, and irrigation on resistance of maize to Fusarium graminearum infection through kernels. Can. J. Plant Pathol. 18, 279—285.
Reid, L. M., and R. C. Sinha, 1998: Maize maturity and the development of Gibberella ear rot symptoms and deoxynivalenol after inoculation. Eur. J. Plant Pathol. 104, 147—154.
Reid, L. M., A. T. Bolton, R. I. Hamilton, T. Woldemariam, and D. E. Mather, 1992a: Effect of silk age on resistance of maize to Fusarium graminearum. Can. J. Plant Pathol. 14, 293—298.
Reid, L. M., R. I. Hamilton, and A. T. Bolton, 1992b: Diallel analysis of resistance in maize to Fusarium graminearum infection via the silk. Can. J. Plant Sci. 2, 915—923.
Reid, L. M., D. Spaner, D. E. Mather, A. T. Bolton, and R. I. Hamilton, 1993: Resistance of maize hybrids and inbreds following silk inoculation with three isolates of Fusarium graminearum. Plant Dis. 77, 1248—1251.
Reid, L. M., R. E. Hamilton, and D. E. Mather, 1996a: Screening Maize for Resistance to Gibberella Ear Rot. Publication 1996–5E, Agriculture and Agri-Food Canada, Technical Bulletin, Ottawa, ON, Canada, 62 pp.
Reid, L. M., D. W. Stewart, and R. I. Hamilton, 1996b: A 4-year study of the association between Gibberella ear rot severity and deoxynivalenol concentration. J. Phytopathol. – Phytopathologische Zeitschrift 144, 431—436.
Reid, L. M., R. W. Nicol, T. Ouellet, M. Savard, J. D. Miller, J. C. Young, D. W. Stewart, and A. W. Schaafsma, 1999: Interaction of Fusarium graminearum and F. moniliforme in maize ears: disease progress, fungal biomass, and mycotoxin accumulation. Phytopathology 89, 1028—1037.
Reid, L. M., X. Zhu, M. E. Savard, R. C. Sinha, and B. Vigier, 2000: Preharvest accumulation of deoxynivalenol in sweet corn ears inoculated with Fusarium graminearum. Food Addit. Contam. A 17, 689—701.
Reid, L. M., G. McDiarmid, A. J. Parker, T. Woldemariam, and R. I. Hamilton, 2001a: CO388 and CO389 corn inbred lines. Can. J. Plant Sci. 81, 457—459.
Reid, L. M., G. McDiarmid, A. J. Parker, T. Woldemariam, and R. I. Hamilton, 2001b: CO430, CO431 and CO432 corn inbred lines. Can. J. Plant Sci. 81, 283—284.
Reid, L. M., T. Woldemariam, X. Zhu, D. W. Stewart, and A. W. Schaafsma, 2002: Effect of inoculation time and point of entry on disease severity in Fusarium graminearum, Fusarium verticillioides, or Fusarium subglutinans inoculated maize ears. Can. J. Plant Pathol. 24, 162—167.
Reid, L. M., G. McDiarmid, A. J. Parker, and T. Woldemariam, 2003: CO441 corn inbred line. Can. J. Plant Sci. 83, 79—80.
Reid, L. M., C. X. Zhu, C. A. Parker, and C. W. Yan, 2009: Increased resistance to Ustilago zeae and Fusarium verticillioides in maize inbred lines bred for Fusarium graminearum resistance. Euphytica 165, 567—578.
Reinprecht, Y., X. G. Wu, S. Yan, L. Labey, E. Dasilva, J. C. Martin, and K. P. Pauls, 2008: A microarray-based approach for identifying genes for resistance to Fusarium graminearum in maize (Zea mays L.). Cereal Res. Commun. 36(Suppl B), 253—259.
Robertson, L. A., C. E. Kleinschmidt, D. G. White, G. A. Payne, C. M. Maragos, and J. B. Holland, 2006: Heritabilities and correlations of Fusarium ear rot resistance and fumonisin contamination resistance in two maize populations. Crop Sci. 46, 353—361. and Erratum, 1420.
Robertson-Hoyt, L. A., M. P. Jines, P. J. Balint-Kurti, C. E. Kleinschmidt, D. G. White, G. A. Payne, C. M. Maragos, T. L. Molnar, and J. B. Holland, 2006c: QTL mapping for Fusarium ear rot and fumonisin contamination resistance in two maize populations. Crop Sci. 46, 1734—1743.
Robertson-Hoyt, L. A., C. E. Kleinschmidt, D. G. White, G. A. Payne, C. M. Maragos, and J. B. Holland, 2007a: Relationships of resistance to Fusarium ear rot and fumonisin contamination with agronomic performance of maize. Crop Sci. 47, 1770—1778.
Robertson-Hoyt, L. A., J. Betra´ n, G. A. Payne, D. G. White, T. Isakeit, C. M. Maragos, T. L. Molna´ r, and J. B. Holland, 2007b: Relationships among resistances to Fusarium and Aspergillus ear rots and contamination by fumonisin and aflatoxin in maize. Phytopathology 97, 311—317.
Sampietro, D. A., M. A. Vattuone, D. A. Presello, C. M. Fauguel, and C. A. N. Catalan, 2009: The pericarp and its surface wax layer in maize kernels as resistance factors to fumonisin accumulation by Fusarium verticillioides. Crop Prot., 28, 196—200.
Sass, V., J. Milles, J. Kramer, and A. Prange, 2007: Competitive interactions of Fusarium graminearum and Alternaria alternata in vitro in relation to deoxynivalenol and zearalenone production. J. Food Agric. Environ. 5, 257—261.
Schaafsma, A. W., R. W. Nicol, and L. M. Reid, 1997: Evaluating commercial maize hybrids for resistance to Gibberella ear rot. Eur. J. Plant Pathol. 103, 737—746.
Schjoth, J. E., A. M. Tronsmo, and L. Sundheim, 2008: Resistance to Fusarium verticillioides in 20 Zambian maize hybrids. J. Phytopathol. 156, 470—479.
Schjoth, J. E., A. Visconti, and L. Sundheim, 2009: Fumonisins in maize in relation to climate, planting time and hybrids in two agroecological zones in Zambia. Mycopathologia 167, 209—219.
Seifert, K. A., T. Auki, R. Baayen, D. Brayford, L. W. Burgess, S. Chulze, A. Gams, D. Geiser, J. de Gruyter, J. Leslie, A. Logrieco, W. F. O. Marasas, H. Nirenberg, K. ODonnell, J. Rheeder, G. J. Samuels, B. A. Summerell, U. Thrane, and C. Waalwijk, 2004: The name Fusarium moniliforme should no longer be used. Mycol. Res. 107, 643—644.
Sekhon, R. S., G. Kuldau, M. Mansfield, and S. Chopra, 2006: Characterization of Fusarium-induced expression of flavonoids and PR genes in maize. Physiol. Mol. Plant Pathol. 69, 109—117.
Silva, E., E. A. Mora, A. Medina, J. Vasquez, D. Valdez, D. L. Danial, and J. E. Parlevliet, 2007: Fusarium ear rot and how to screen for resistance in open pollinated maize in the Andean regions. Euphytica 153, 329—337.
Snijders, C. H. A., and F. A. van Eeuwijk, 1991: Genotype-strain interactions for resistance to Fusarium head blight caused by Fusarium culmorum in winter wheat. Theor. Appl. Genet. 81, 239—244.
Sprague, G. F., 1977: Corn and Corn Improvement. No. 18. in the series Agronomy, American Society of Agronomy, Inc. Publisher, Madison, WI, USA, 774 pp.
Sutton, J. C., 1982: Epidemiology of wheat head blight and maize ear rot caused by Fusarium graminearum. Can. J. Plant Pathol. 4, 195—209.
Sutton, J. C., and W. Baliko, 1981: Methods for quantifying resistance to Gibberella zeae in maize ears. Can. J. Plant Pathol. 3, 26—32.
Szuts, P., A ´ . Mesterha´ zy, G. Falkay, and T. Barto´ k, 1997: Early telarche symptoms in children and their relations to zearalenone contamination in foodstuffs. Cereal Res. Commun. 25, 429—436.
Takegami, S., and K. Sasai, 1970: Investigations on resistance of wheat varieties to Gibberella zeae after particular inoculation techniques. X. Experiments on improved inoculation methods involving conidiospores and hyphae. Proc. Crop. Sci. Soc. Jpn., 39, 1—6.
Tanaka, T., A. Hasegawa, S. Yamamoto, U.-S. Lee, Y. Surgiura, and Y. Ueno, 1988: Worldwide contamination of cereals by the Fusarium mycotoxins nivalenol, deoxynivalenol, and zearalenone. 1. Survey of 19 countries. J. Agric. Food. Chem. 36, 979—983.
Toldi, E., T. Barto´ k, M. Varga, A. Szekeres, B. To´ th, and A ´ . Mesterha´ zy, 2008: The role of breeding in reducing mycotoxin contamination in corn. Cereal Res. Commun. 36(Suppl B), 175—177.
Ullstrup, A. J., 1953: Several Ear Rots of Corn. U.S. Department of Agriculture Y. B., the headquarter of USDA research center, Beltsville, USDA. 390—392.
Ullstrup, A. J., 1970: Methods of inoculating corn ears with Gibberella zeae and Diplodia maydis. Plant Dis. Reporter 54, 658—662.
Ullstrup, A. J., 1977: Corn diseases. In: G. F. Sprague (ed.), Corn and Corn Improvement, 391—500. No. 18. in the series AGRONOMY. American Society of Agronomy, Inc. Publisher, Madison, WI, USA. 774 pp.
Vesonder, R. F., A. Ciegler, H. R. Burmeister, and A. H. Jensen, 1979: Acceptance by swine and rats of corn amended with trichothecenes. Appl. Environ. Microbiol. 38, 344—346.
Vesonder, R. F., J. Ellis, and W. K. Rohwedder, 1981: Elaboration of vomitoxin and zearalenone by Fusarium isolates and the biological activity of Fusarium-produced toxins. Appl. Environ. Microbiol. 42, 1132—1134.
Vigier, B., L. M. Reid, L. M. Dwyer, D. W. Stewart, R. C. Sinha, J. T. Arnason, and G. Butler, 2001: Maize resistance to Gibberella ear rot: symptoms, deoxynivalenol, and yield. Can. J. Plant Pathol. 23, 99—105.
Voss, K. A., J. B. Gelineau-van Waes, and R. T. Riley, 2006: Fumonisins: current research trends in developmental toxicology. Mycotoxin Res. 22, 61—69.
Waalwijk, C., R. van der Heide, I. de Vries, T. van der Lee, C. Schoen, G. Costrel-de Corainville, I. Hauser-Hahn, P. Kastelein, J. Kohl, P. Lonnet, T. Demarquet, and G. H. J. Kema, 2004: Quantitative detection of Fusarium species in wheat using TaqMan. Eur. J. Plant Pathol. 110, 481—494.
Warfield, C. Y., and R. M. Davis, 1996: Importance of husk covering on the susceptibility of corn hybrids to Fusarium ear rot. Plant Dis., 80, 208—210.
White, D. G., 1999: Compendium of Corn Diseases, 3rd edn. APS Press, St. Paul, MN, ISBN 978-0-89054-234-7, 128 pages.
Wilde, F., V. Korzun, E. Ebmeyer, H. H. Geiger, and T. Miedaner, 2007: Comparison of phenotypic and marker-based selection for Fusarium head blight resistance and DON content in spring wheat. Mol. Breed. 19, 357—370.
Williams, W. P., 2006: Breeding for resistance to aflatoxin accumulation in maize. Mycotoxin Res. 22, 27—32.
Williams, W. P., and G. L. Windham, 2009: Diallel analysis of fumonisin accumulation in maize. Field Crops Res. 114, 324—326.
Wu, F., 2006: Mycotoxin reduction in Bt corn: potential economic, health, and regulatory impacts. Transgenic Res. 15, 277—289.
Xiang, K., L. M. Reid, and X. Zhu. 2010a: Relationship among kernel drydown rates, environmental factors and resistance to Gibberella ear rot, Fusarium ear rot and common smut of corn. Joint Annual Meeting of the Canadian Phytopathological Society and the Pacific Division of the American Phytopathological Society, June 19–23, Vancouver, BC.
Xiang, K., Z. M. Zhang, L. M. Reid, X. Y. Zhu, G. S. Yuan, and G. T. Pan, 2010b: A meta-analysis of QTL associated with ear rot resistance in maize. Maydica 55, 281—290.
Xu, X.-M., D. W., Parry., P. Nicholson, M. A. Thomsett, D. Simpson, S. G. Edwards, B. M. Cooke, F. M. Doohan, S. Monaghan, A. Moretti, G. Tocco, G. Mule, L. Hornok, E. Be´ ki, J. Tatnell, and A. Ritieni, 2008: Within-field variability of Fusarium head blight pathogens and their associated mycotoxins. Eur. J. Plant Pathol. 120, 21—34.
Young, H. C., Jr, 1943: The toothpick method of inoculating corn for ear and stalk rots. Phytopathology 33, 16. (Abstr.)
Yuan, J., M. Liakat Ali, J. Taylor, J. Liu, G. Sun, W. Liu, P. Masilimany, A. Gulati-Sakhuja, and K. P. Pauls, 2008: A guanylyl cyclase-like gene is associated with Gibberella ear rot resistance in maize (Zea mays L.). Theor. Appl. Genet. 116, 465—479.
Zhang, Y., Y.-E. Choi, X. Zou, and J.-R. Xu, 2011: The FvMK1 mitogen-activated protein kinase gene regulates conidiation, pathogenesis, and fumonisin production in Fusarium verticillioides. Fungal Genet. Biol. 48, 71—79.
Related topics
Authors:
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post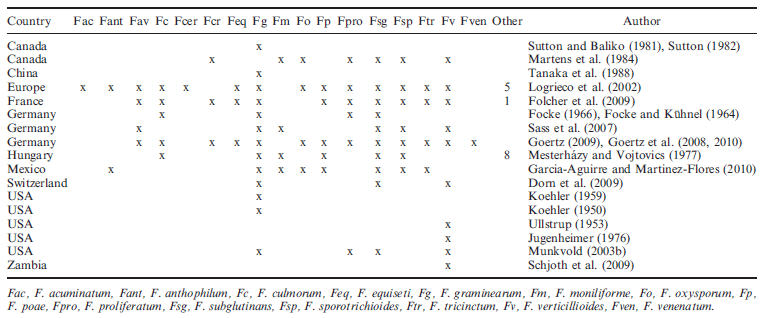
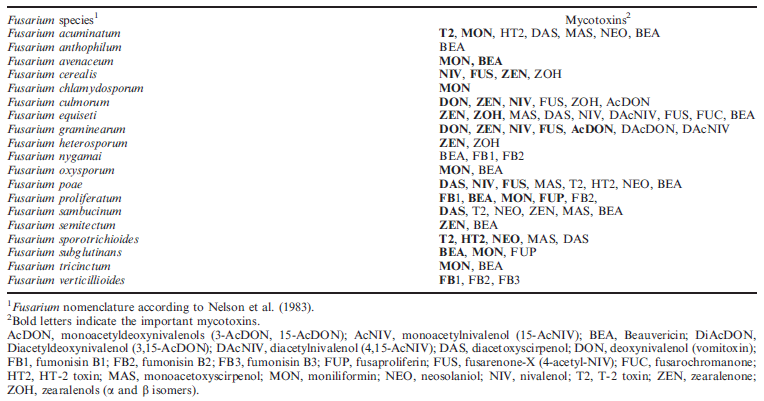






.jpg&w=3840&q=75)