Nutraceuticals in equine nutrition
Nutraceuticals: their emerging role in equine nutrition
Published: October 25, 2006
By: BRIAN D. NIELSEN - Alltech Inc.
Horse nutrition differs substantially from the feeding of other livestock species. Whereas in other species, producers ask the question “will it help?” before feeding a supplement, individuals in the horse industry tend to ask “will it hurt?” when making a decision as to whether or not to feed a supplement. As a result, the practice of feeding nutraceuticals is relatively common in the equine industry.
What are nutraceuticals?
The Food and Drug Administration (FDA) requires a ‘food’ to provide taste, aroma or nutritive value (FDA, 1995). In contrast, the supplement industry has embraced the definition of a nutraceutical as ‘any non-toxic food component that has scientifically proven health benefits, including disease treatment or prevention’ (Boothe, 1997). The inclusion of ‘disease treatment’ in that definition results in controversy. As the FDA is charged with approving medications before they can legally be sold or used, by including ‘disease treatment’ in the definition, it can be argued that the FDA should have a role in regulating many supplements.
However, the Dietary Supplement Health and Education Act of 1994 allows beneficial claims to be made when the claim is made for prevention or treatment of a classic nutrient deficiency (Jones, 1997). By avoiding being classified as a drug, the amount of regulations surrounding a supplement decreases substantially. There are both advantages and disadvantages to having a substance classified as a nutraceutical rather than a drug even if it has purported medicinal properties. By being classified as a nutraceutical, less testing of the substance is required, thus reducing cost to the manufacturer and shortening the time before a product can be marketed. By avoiding the extensive testing required by the FDA to prove both efficacy and safety, the cost to the consumer is typically less. Additionally, the consumer will tend to have a greater selection of products and will have access to them sooner.
However, there is a trade-off. Without any regulations controlling the sale of nutraceuticals, the purity of products sold should be questioned. Situations have arisen in which the analyses of some products have revealed a total lack of alleged active ingredient (Adebowale et al., 2000). Other than purchasing a product through a reputable company, most consumers will have no guarantee as to the actual product contents. Furthermore, there is no guarantee that the supplement is safe. Ideally, long-term feeding studies should be conducted to make sure no harm to the animal results from consuming a product. Finally, without the requirement of proper testing, many nutraceuticals are sold without ever having demonstrated efficacy in a scientifically valid manner. Without such testing, a certain degree of cynicism is warranted.
Difficulty in proving efficacy
Proving the efficacy of a nutraceutical can be exceedingly difficult. To do so, the hypothesis that a supplement has a desirable effect must be tested in a controlled study. If the results show that animals receiving the treatment reacted statistically differently from a control group that did not receive the treatment, the hypothesis is accepted. If it is not possible to demonstrate a statistical difference between animals serving as controls and those receiving a treatment, the hypothesis is rejected and it is concluded that the treatment was not efficacious. However, an improperly designed study can almost guarantee that no difference can be detected and the rejection of the hypothesis might be in error (Hancock, 1992). Such improperly designed studies are likely to occur more often in equine studies than in other livestock species due to the difficulty in obtaining sufficient numbers of research animals.
In equine research, most studies seem to have approximately 12 to 16 animals with between six to eight horses being assigned to each treatment. In contrast, poultry researchers think nothing of having 1000 or 10,000 birds in a study. However, the 1000-bird study often will cost the same as a 14 horse study lasting the same length of time (K. Roberson, personal communication). To be able to have such a large number of equine subjects would be economically infeasible; but without a sufficient number of research animals, subtle differences between treatments may not be detected.
In food animal species, one could argue that if it is not possible to detect the difference, it is likely not economically important. In dairy nutrition, changing the nutrient composition of the ration can result in detectable changes in milk production within just a few days (Etchebarne, 2002). However, improvements in equine athletic performance can be much more subtle and harder to detect, although this does not automatically make the improvement less economically important. Using Quarter Horse racing as an example, only a fraction of a second in a final race time can make a tremendous difference in earning potential. For instance, in the 2001 All American Futurity with a purse of $1,996,368, the time for ninth fastest qualifier was initially posted as 21.745 seconds (Speedhorse, 2001). Though that time was later found to be incorrect and changed to a slightly faster time, compare it to the time of the 11th place qualifier, Dunit Runnin, who won his trial heat in a time of 21.744 seconds. Since only the top 10 horses compete in the finals of this $2 million race, 1/1000th of a second could prove to be very economically significant! In a race lasting just under 22 seconds, this difference in time is extremely small but can have a huge impact on a horse’s earning potential.
If a supplement was able to decrease the race time of a horse by this small fraction of a time, it would be virtually impossible to detect that difference through scientific testing. There are too many other variables that would have a greater impact such as rider, track conditions, wind conditions and many more. Many trainers would gladly pay for a supplement that would have even a small effect. As one trainer put it, “I don’t know if it works, but if it does, I want to have the same advantage other trainers have.”
Keeping in mind the difficulty in testing many of these nutraceuticals, it is also quite difficult to say that a product does not work. Even if tests have not shown any differences, there is a good possibility that not a sufficient number of horses were used in the study to detect a difference if the effect was quite small. At the same time, one should be cautioned against listening to anecdotal evidence. Individuals in the equine industry are willing to listen to another trainer or veterinarian who claims that a product worked based upon the ‘results’ of feeding it to a few horses in a non-controlled setting. What these individuals fail to recognize is that often other items are changed at the same time feeding the nutraceutical began. Some of these other changes may have been instrumental in causing a difference. Alternatively, if the nutraceutical is being fed in order to facilitate healing of an injury, potentially it is just time that causes the response.
Bearing these various factors in mind, it is not possible to say whether many of the nutraceuticals actually work. However, it is possible to review the studies that have been done, evaluate the quality of the research, and also explore possible mechanisms through which a nutraceutical might work. The following list of nutraceuticals is obviously not comprehensive, but covers a few of the more popular products on the market.
Supplements for joint health
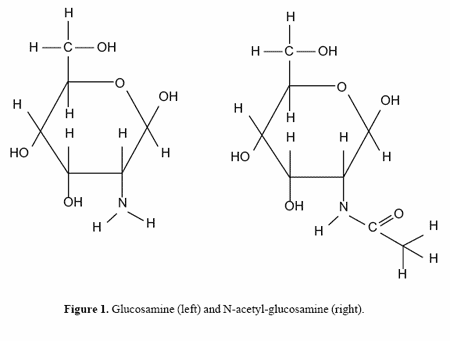
Facilitating joint repair and improving joint health are some of the most common uses of nutraceuticals in both horse and human nutrition. Nutraceuticals, such as glucosamine and chondroitin sulfate, offer possible chondro-protective effects against joint injury (Setnikar et al., 1986). Glucosamine is an amino sugar with the amide nitrogen of glutamine transferred to the C-2 carbon of fructose. In articular cartilage, it is not found in the free form, but is found as N-acetyl-glucosamine that has been formed from glucosamine (Figure 1). Chondroitin sulfate is a glycosaminoglycan found naturally in the extracellular matrix of articular cartilage (Uebelhart et al., 1998). In order for either to be effective, both must be absorbed. Setnikar et al. (1986) demonstrated that in dogs, radiolabelled glucosamine sulfate is quickly absorbed and relatively active uptake of the compound occurs in articular cartilage. However, in vivo data are sparse and clinical trials have been performed primarily in humans (McCarthy, 1989).
However, an improvement in lameness grade, flexion test and stride length has been reported in horses with degenerative joint disease that received a glucosamine-chondroitin sulfate compound (Hanson et al., 1997). Unfortunately, no horses with osteoarthritis were used as untreated controls, so the results are suspect. When feeding glucosamine as a chondro-protective agent to clinically normal horses, benefits seem to be minimal (Nielsen et al., 1998).
One of the more convincing in vivo studies that has reported positive effects of oral consumption of these compounds was a randomized, double-blind, placebo-controlled pilot study. The authors reported that the combination of glucosamine, chondroitin sulfate and manganese ascorbate decreased knee osteoarthritis symptoms in humans as indicated by disease scores, patient assessment of treatment effect, visual analog scale for pain and physical examination scores (Leffler et al., 1999). Also, chondroitin sulfate has been shown to decrease pain and increase overall mobility in humans with osteoarthritic knees (Uebelhart et al., 1998).
In vitro studies also show promising results. Such research suggests that glucosamine increases the synthetic activity of chondrocytes, which could improve cartilage repair (Bassleer et al., 1992).
Likewise, glucosamine has been found to regulate several important metabolic activities of osteoarthritic chondrocytes including an increase in protein synthesis (Piperno et al., 2000). Such activity may be helpful in stimulating matrix synthesis – a potentially important part of healing (Algner et al., 1997). Furthermore, both glucosamine and a similar hexosamine, mannosamine, were reported to inhibit cartilage damage when treated with lipopolysaccharide in vitro (Mello et al., 2001). In addition, Sandy et al. (1998) demonstrated the ability of glucosamine to inhibit aggrecanase activity in chondrocytes. Furthermore, in vitro studies with human chondrocytes treated with interleukin-1ß demonstrated the protective effects of chondroitin sulfate (Nerucci et al., 2000).
Another compound commonly fed to horses to promote joint health is methylsulfonlymethane (MSM). This stable, odorless metabolite of dimethlysulfoxide (DMSO) supposedly has no “exceptional activity, in vivo, where biological normalcy is present” and it is reputed to simply return an abnormal state to normal health (Metcalf, 1983). When searching for a mechanism of action, many researchers believe that the primary benefit of feeding MSM is to restore proper sulfur concentrations into the diet. Such a role may explain why some people believe it is useful in healing damaged collagen found in connective tissue and may be useful in the formation of chondroitin sulfate.
Bone formation
Despite silicon (Si) being the second most common element of the Earth’s crust (Carlisle, 1972), surprisingly little is known about the nutritional importance of Si in the diets of mammalian species. The relative abundance of Si makes the deficiency of this element difficult to achieve. That being said, the American Institute of Nutrition recently reformulated their published formulas of purified diets for experimental rodents and made the decision to include Si as a required nutrient (Reeves, 1997). This change was brought about as a result of research demonstrating that Si can interact with other nutrients for apparent beneficial effects (Nielsen, 1991). Though the National Research Council (1989) has not specified a requirement for Si in horses, benefits have been demonstrated by feeding a bioavailable Si source.
As part of a large, blinded FDA-controlled study, Nielsen et al. (1993) reported dramatic decreases in injury rates to racing Quarter Horses fed a supplemental Si source – sodium zeolite A. The control group not receiving supplemental Si had more horses experience injuries that caused removal from training than horses that were able to complete the study. All three treatment groups receiving the Si source had more horses complete the study without injury. Additionally, horses supplemented with the medium and high doses of Si were able to train and race nearly twice the distance before experiencing an injury than did the control group. Recent studies have demonstrated increased osteocalcin (marker of bone formation) concentrations in broodmares during the first 45 days after supplementation commencing at parturition (Lang et al., 2001b) and decreased carboxyterminal pyridinoline cross-linked telopeptide region of type I collagen (ICTP – marker of bone resorption) concentrations by day 45 in yearlings after the beginning of supplementation (Lang et al., 2001a).
Ergogenic agents
Several supplements are available that purport to increase work capacity by reducing fatigue symptoms. The role of branch-chained amino acids (BCAA) in endurance exercise in the horse is an area that has received attention by several research groups (Glade, 1991; Casini et al., 2000, Trottier et al., 2002). Unconditioned Quarter Horses orally receiving BCAA and undergoing intense race training over nine weeks had reduced lactate concentrations and delayed onset of lactate concentration increases compared to nonsupplemented horses (Glade, 1991).
In contrast, conditioned Standardbreds did not have reduced plasma lactate concentrations after supplementation (Casini et al., 2000). In humans, performance in a 100-km time-trial bike race was not enhanced through supplementation (Madsen et al., 1996) but BCAA supplementation did increase endurance performance in untrained rats (Calders et al., 1997). It is possible, based upon these studies, that the capacity of muscle to oxidize BCAA in the conditioned horse is higher compared to the unconditioned horse (Trottier et al., 2002). If true, oral administration of BCAA would not be of much benefit to the conditioned horse.
Carnitine is an essential cofactor involved in the oxidation of long-chain fatty acids by aiding in transport across the inner mitochondrial membrane (Snow, 1992). Because of its role in fatty acid oxidation, it has been proposed that carnitine may be helpful in endurance exercise. Specifically, when high fatty acid turnover is required, carnitine availability may be limiting. In humans, results of carnitine supplementation have been mixed, though Williams (1992) suggests that supplementation does not improve energy utilization or endurance and there is not enough evidence to support its use in humans. In the horse, carnitine is poorly absorbed after oral administration (Foster and Harris, 1989) and has failed to increase equine muscle carnitine content (Foster et al., 1988). High concentrations of carnitine are found in both skeletal and cardiac muscle but not in plants (Bremer, 1983). Thus, since horses eat herbaceous diets, they likely have not evolved to adequately absorb dietary carnitine.
Oral administration of creatine (20 to 30 g/day for up to 5 days) has been shown to increase muscle total creatine pool in humans (Harris et al., 1992). In contrast to carnitine supplementation, which is used to increase endurance, the increase in creatine has been reported to enhance sprinting performance in man (Harris et al., 1993). However, it is questionable if the same results would be seen with horses.
Bee pollen is another ergogenic agent fed to racehorses for the purpose of added ‘energy and stamina’. Citing the German research scientist Dr. H. Wanderka, Iannuzzi (1994) reported an equine study that indicated horses receiving bee pollen were more balanced, enthusiastic about performing their work and showed less exhaustion. Unfortunately, details by which the quality of the study can be judged are not available. Other equine studies, controlled or otherwise, have not been found. In spite of this, many trainers purchase and use this supplement. The one study that was found involved feeding bee pollen as the sole source of nutrition instead of as a supplement to improve performance. By feeding rats a diet consisting solely of bee pollen, Liebelt and Calcagnetti (1999) concluded that bee pollen contains all the essential nutritional elements to provide normal rat growth and development. This supports the argument that bee pollen is a good source of nutrients but does not clarify whether it can actually improve the performance of an equine athlete.
What are nutraceuticals?
The Food and Drug Administration (FDA) requires a ‘food’ to provide taste, aroma or nutritive value (FDA, 1995). In contrast, the supplement industry has embraced the definition of a nutraceutical as ‘any non-toxic food component that has scientifically proven health benefits, including disease treatment or prevention’ (Boothe, 1997). The inclusion of ‘disease treatment’ in that definition results in controversy. As the FDA is charged with approving medications before they can legally be sold or used, by including ‘disease treatment’ in the definition, it can be argued that the FDA should have a role in regulating many supplements.
However, the Dietary Supplement Health and Education Act of 1994 allows beneficial claims to be made when the claim is made for prevention or treatment of a classic nutrient deficiency (Jones, 1997). By avoiding being classified as a drug, the amount of regulations surrounding a supplement decreases substantially. There are both advantages and disadvantages to having a substance classified as a nutraceutical rather than a drug even if it has purported medicinal properties. By being classified as a nutraceutical, less testing of the substance is required, thus reducing cost to the manufacturer and shortening the time before a product can be marketed. By avoiding the extensive testing required by the FDA to prove both efficacy and safety, the cost to the consumer is typically less. Additionally, the consumer will tend to have a greater selection of products and will have access to them sooner.
However, there is a trade-off. Without any regulations controlling the sale of nutraceuticals, the purity of products sold should be questioned. Situations have arisen in which the analyses of some products have revealed a total lack of alleged active ingredient (Adebowale et al., 2000). Other than purchasing a product through a reputable company, most consumers will have no guarantee as to the actual product contents. Furthermore, there is no guarantee that the supplement is safe. Ideally, long-term feeding studies should be conducted to make sure no harm to the animal results from consuming a product. Finally, without the requirement of proper testing, many nutraceuticals are sold without ever having demonstrated efficacy in a scientifically valid manner. Without such testing, a certain degree of cynicism is warranted.
Difficulty in proving efficacy
Proving the efficacy of a nutraceutical can be exceedingly difficult. To do so, the hypothesis that a supplement has a desirable effect must be tested in a controlled study. If the results show that animals receiving the treatment reacted statistically differently from a control group that did not receive the treatment, the hypothesis is accepted. If it is not possible to demonstrate a statistical difference between animals serving as controls and those receiving a treatment, the hypothesis is rejected and it is concluded that the treatment was not efficacious. However, an improperly designed study can almost guarantee that no difference can be detected and the rejection of the hypothesis might be in error (Hancock, 1992). Such improperly designed studies are likely to occur more often in equine studies than in other livestock species due to the difficulty in obtaining sufficient numbers of research animals.
In equine research, most studies seem to have approximately 12 to 16 animals with between six to eight horses being assigned to each treatment. In contrast, poultry researchers think nothing of having 1000 or 10,000 birds in a study. However, the 1000-bird study often will cost the same as a 14 horse study lasting the same length of time (K. Roberson, personal communication). To be able to have such a large number of equine subjects would be economically infeasible; but without a sufficient number of research animals, subtle differences between treatments may not be detected.
In food animal species, one could argue that if it is not possible to detect the difference, it is likely not economically important. In dairy nutrition, changing the nutrient composition of the ration can result in detectable changes in milk production within just a few days (Etchebarne, 2002). However, improvements in equine athletic performance can be much more subtle and harder to detect, although this does not automatically make the improvement less economically important. Using Quarter Horse racing as an example, only a fraction of a second in a final race time can make a tremendous difference in earning potential. For instance, in the 2001 All American Futurity with a purse of $1,996,368, the time for ninth fastest qualifier was initially posted as 21.745 seconds (Speedhorse, 2001). Though that time was later found to be incorrect and changed to a slightly faster time, compare it to the time of the 11th place qualifier, Dunit Runnin, who won his trial heat in a time of 21.744 seconds. Since only the top 10 horses compete in the finals of this $2 million race, 1/1000th of a second could prove to be very economically significant! In a race lasting just under 22 seconds, this difference in time is extremely small but can have a huge impact on a horse’s earning potential.
If a supplement was able to decrease the race time of a horse by this small fraction of a time, it would be virtually impossible to detect that difference through scientific testing. There are too many other variables that would have a greater impact such as rider, track conditions, wind conditions and many more. Many trainers would gladly pay for a supplement that would have even a small effect. As one trainer put it, “I don’t know if it works, but if it does, I want to have the same advantage other trainers have.”
Keeping in mind the difficulty in testing many of these nutraceuticals, it is also quite difficult to say that a product does not work. Even if tests have not shown any differences, there is a good possibility that not a sufficient number of horses were used in the study to detect a difference if the effect was quite small. At the same time, one should be cautioned against listening to anecdotal evidence. Individuals in the equine industry are willing to listen to another trainer or veterinarian who claims that a product worked based upon the ‘results’ of feeding it to a few horses in a non-controlled setting. What these individuals fail to recognize is that often other items are changed at the same time feeding the nutraceutical began. Some of these other changes may have been instrumental in causing a difference. Alternatively, if the nutraceutical is being fed in order to facilitate healing of an injury, potentially it is just time that causes the response.
Bearing these various factors in mind, it is not possible to say whether many of the nutraceuticals actually work. However, it is possible to review the studies that have been done, evaluate the quality of the research, and also explore possible mechanisms through which a nutraceutical might work. The following list of nutraceuticals is obviously not comprehensive, but covers a few of the more popular products on the market.
Supplements for joint health
Facilitating joint repair and improving joint health are some of the most common uses of nutraceuticals in both horse and human nutrition. Nutraceuticals, such as glucosamine and chondroitin sulfate, offer possible chondro-protective effects against joint injury (Setnikar et al., 1986). Glucosamine is an amino sugar with the amide nitrogen of glutamine transferred to the C-2 carbon of fructose. In articular cartilage, it is not found in the free form, but is found as N-acetyl-glucosamine that has been formed from glucosamine (Figure 1). Chondroitin sulfate is a glycosaminoglycan found naturally in the extracellular matrix of articular cartilage (Uebelhart et al., 1998). In order for either to be effective, both must be absorbed. Setnikar et al. (1986) demonstrated that in dogs, radiolabelled glucosamine sulfate is quickly absorbed and relatively active uptake of the compound occurs in articular cartilage. However, in vivo data are sparse and clinical trials have been performed primarily in humans (McCarthy, 1989).
However, an improvement in lameness grade, flexion test and stride length has been reported in horses with degenerative joint disease that received a glucosamine-chondroitin sulfate compound (Hanson et al., 1997). Unfortunately, no horses with osteoarthritis were used as untreated controls, so the results are suspect. When feeding glucosamine as a chondro-protective agent to clinically normal horses, benefits seem to be minimal (Nielsen et al., 1998).
One of the more convincing in vivo studies that has reported positive effects of oral consumption of these compounds was a randomized, double-blind, placebo-controlled pilot study. The authors reported that the combination of glucosamine, chondroitin sulfate and manganese ascorbate decreased knee osteoarthritis symptoms in humans as indicated by disease scores, patient assessment of treatment effect, visual analog scale for pain and physical examination scores (Leffler et al., 1999). Also, chondroitin sulfate has been shown to decrease pain and increase overall mobility in humans with osteoarthritic knees (Uebelhart et al., 1998).
In vitro studies also show promising results. Such research suggests that glucosamine increases the synthetic activity of chondrocytes, which could improve cartilage repair (Bassleer et al., 1992).
Likewise, glucosamine has been found to regulate several important metabolic activities of osteoarthritic chondrocytes including an increase in protein synthesis (Piperno et al., 2000). Such activity may be helpful in stimulating matrix synthesis – a potentially important part of healing (Algner et al., 1997). Furthermore, both glucosamine and a similar hexosamine, mannosamine, were reported to inhibit cartilage damage when treated with lipopolysaccharide in vitro (Mello et al., 2001). In addition, Sandy et al. (1998) demonstrated the ability of glucosamine to inhibit aggrecanase activity in chondrocytes. Furthermore, in vitro studies with human chondrocytes treated with interleukin-1ß demonstrated the protective effects of chondroitin sulfate (Nerucci et al., 2000).
Another compound commonly fed to horses to promote joint health is methylsulfonlymethane (MSM). This stable, odorless metabolite of dimethlysulfoxide (DMSO) supposedly has no “exceptional activity, in vivo, where biological normalcy is present” and it is reputed to simply return an abnormal state to normal health (Metcalf, 1983). When searching for a mechanism of action, many researchers believe that the primary benefit of feeding MSM is to restore proper sulfur concentrations into the diet. Such a role may explain why some people believe it is useful in healing damaged collagen found in connective tissue and may be useful in the formation of chondroitin sulfate.

Bone formation
Despite silicon (Si) being the second most common element of the Earth’s crust (Carlisle, 1972), surprisingly little is known about the nutritional importance of Si in the diets of mammalian species. The relative abundance of Si makes the deficiency of this element difficult to achieve. That being said, the American Institute of Nutrition recently reformulated their published formulas of purified diets for experimental rodents and made the decision to include Si as a required nutrient (Reeves, 1997). This change was brought about as a result of research demonstrating that Si can interact with other nutrients for apparent beneficial effects (Nielsen, 1991). Though the National Research Council (1989) has not specified a requirement for Si in horses, benefits have been demonstrated by feeding a bioavailable Si source.
As part of a large, blinded FDA-controlled study, Nielsen et al. (1993) reported dramatic decreases in injury rates to racing Quarter Horses fed a supplemental Si source – sodium zeolite A. The control group not receiving supplemental Si had more horses experience injuries that caused removal from training than horses that were able to complete the study. All three treatment groups receiving the Si source had more horses complete the study without injury. Additionally, horses supplemented with the medium and high doses of Si were able to train and race nearly twice the distance before experiencing an injury than did the control group. Recent studies have demonstrated increased osteocalcin (marker of bone formation) concentrations in broodmares during the first 45 days after supplementation commencing at parturition (Lang et al., 2001b) and decreased carboxyterminal pyridinoline cross-linked telopeptide region of type I collagen (ICTP – marker of bone resorption) concentrations by day 45 in yearlings after the beginning of supplementation (Lang et al., 2001a).
Ergogenic agents
Several supplements are available that purport to increase work capacity by reducing fatigue symptoms. The role of branch-chained amino acids (BCAA) in endurance exercise in the horse is an area that has received attention by several research groups (Glade, 1991; Casini et al., 2000, Trottier et al., 2002). Unconditioned Quarter Horses orally receiving BCAA and undergoing intense race training over nine weeks had reduced lactate concentrations and delayed onset of lactate concentration increases compared to nonsupplemented horses (Glade, 1991).
In contrast, conditioned Standardbreds did not have reduced plasma lactate concentrations after supplementation (Casini et al., 2000). In humans, performance in a 100-km time-trial bike race was not enhanced through supplementation (Madsen et al., 1996) but BCAA supplementation did increase endurance performance in untrained rats (Calders et al., 1997). It is possible, based upon these studies, that the capacity of muscle to oxidize BCAA in the conditioned horse is higher compared to the unconditioned horse (Trottier et al., 2002). If true, oral administration of BCAA would not be of much benefit to the conditioned horse.
Carnitine is an essential cofactor involved in the oxidation of long-chain fatty acids by aiding in transport across the inner mitochondrial membrane (Snow, 1992). Because of its role in fatty acid oxidation, it has been proposed that carnitine may be helpful in endurance exercise. Specifically, when high fatty acid turnover is required, carnitine availability may be limiting. In humans, results of carnitine supplementation have been mixed, though Williams (1992) suggests that supplementation does not improve energy utilization or endurance and there is not enough evidence to support its use in humans. In the horse, carnitine is poorly absorbed after oral administration (Foster and Harris, 1989) and has failed to increase equine muscle carnitine content (Foster et al., 1988). High concentrations of carnitine are found in both skeletal and cardiac muscle but not in plants (Bremer, 1983). Thus, since horses eat herbaceous diets, they likely have not evolved to adequately absorb dietary carnitine.
Oral administration of creatine (20 to 30 g/day for up to 5 days) has been shown to increase muscle total creatine pool in humans (Harris et al., 1992). In contrast to carnitine supplementation, which is used to increase endurance, the increase in creatine has been reported to enhance sprinting performance in man (Harris et al., 1993). However, it is questionable if the same results would be seen with horses.
Bee pollen is another ergogenic agent fed to racehorses for the purpose of added ‘energy and stamina’. Citing the German research scientist Dr. H. Wanderka, Iannuzzi (1994) reported an equine study that indicated horses receiving bee pollen were more balanced, enthusiastic about performing their work and showed less exhaustion. Unfortunately, details by which the quality of the study can be judged are not available. Other equine studies, controlled or otherwise, have not been found. In spite of this, many trainers purchase and use this supplement. The one study that was found involved feeding bee pollen as the sole source of nutrition instead of as a supplement to improve performance. By feeding rats a diet consisting solely of bee pollen, Liebelt and Calcagnetti (1999) concluded that bee pollen contains all the essential nutritional elements to provide normal rat growth and development. This supports the argument that bee pollen is a good source of nutrients but does not clarify whether it can actually improve the performance of an equine athlete.
A final ergogenic agent to be discussed is dimethylglycine (DMG). An intermediate of choline metabolism, this product is marketed to horse owners and trainers as a method of reducing lactate accumulation in horses (Warren et al., 1999). A 1982 study by Gannon and Kendall reported that greyhounds administered DMG had improved performances compared to a trial run without DMG five to seven days earlier. Unfortunately, the design of that study can be faulted due to the conditioning effect of the dogs having run a few days earlier.
Other confounding variables may also have played a role in the improved performance. Well-controlled studies in humans have not shown consistent ergogenic effects (Bucci, 1993); and in a crossover study using a placebo and DMG, Rose et al. (1989) found no beneficial effects on cardiovascular function or lactate accumulation in the exercising horse. One study finding beneficial effects of DMG administration in horses was done by Levine et al. (1982). Comparing horses given DMG to those not receiving any, blood samples were taken after exercising on six separate days for the treated horses but only on four days for the control horses.
Though treated horses were reported to have lower blood lactic acid concentrations after exercising than the controls, it is interesting to note that DMG did not decrease the lactate concentrations from those recorded at the beginning of the study. If DMG was truly efficacious in lowering blood lactate concentrations, one might expect lower concentrations after horses had been consuming the product for a period of time. This discrepancy was never explained. Also in this study, a major point was that trainers subjectively claimed treated horses were “more aggressive”, had “better appetites and attitudes”, and were able “to recover faster from racing and training than the controls”. As a critical reviewer of a study, one needs to question whether this was just a perceived effect, as the trainers were not blinded to the treatments. All too often if a trainer believes a product will improve the attitude of a horse, that trainer will testify that such a change has occurred.
Conclusions
The nutraceuticals discussed in this review are just a few of those available on the market. Though it would be nice if one could decide conclusively whether a given supplement is efficacious, it is extremely difficult to do so. As can be seen, even if a product appears to work in a given study, if the research design is flawed, the evidence must be viewed with skepticism. Alternatively, if a properly controlled study fails to show an advantage in treated animals compared to control animals, it still does not prove the product did not work if a sufficient number of research subjects was not used. With the possibility of huge sums of money to be won by having only a very small improvement in performance, it is reasonable to expect horse owners and trainers will continue to use nutraceuticals whether their efficacy has been proven or not.
References
Adebowale, A.O., D.S. Cox, Z. Liang and N.D. Eddington. 2000. Analysis of glucosamine and chondroitin sulfate content in marketed products and the Caco-2 permeability of chondroitin sulfate raw materials. J. Amer. Nutraceutical Assoc. 3(1):37-44.
Algner, T., S.I. Vornehm, G. Zeidler, J. Dudhia, K. Von der Mark and M.T. Bayliss. 1997. Suppression of cartilage matrix gene expression in upper zone chondrocytes of osteoarthritic cartilage. Arthritis Rheum. 40:562-569.
Bassleer, C., Y. Henrotin and P. Franchimont. 1992. In vitro evaluation of drugs proposed as chondroprotective agents. Int. J. Tiss. Reac. 14(5):231-241.
Boothe, D.M. 1997. Nutraceuticals in veterinary medicine Part 1: Definitions and regulations. Comp. Cont. Ed. 19(11).
Bremer, J. 1983. Carnitine-metabolism and functions. Physiol. Rev. 63:1420-1480.
Bucci, L. 1993. Nutrients as ergonic aids for sports and exercise. Boca Raton, CRC Press.
Calders, P., J.L. Pannier, D.M. Matthys and E.M. Lacroix. 1997. Pre-exercise branched-chain amino acid administration increases endurance performance in rats. Med. Sci. Sports Exerc. 29(9):1182-1186.
Carlisle, E.M. 1972. Silicon: An essential element for the chick. Science, Pp. 178:619.
Casini, L., D. Gatta, L. Magni and B. Colombani. 2000. Effect of prolonged branched-chain amino acid supplementation on metabolic response to anaerobic exercise in Standardbreds. J. Equine Vet. Sci. 20:120-123.
Etchebarne, M. 2002. Dairy nutrition in the west. Nutrition Seminar. Michigan State University. Feb. 11.
FDA. 1995. FDA’s view of dietary supplement legislation. FDA Vet. May/June.
Foster, C.V.L. and R.C. Harris. 1989. Plasma carnitine concentrations in the horse following oral supplementation using a triple dose regime. Equine Vet. J. 21:376-377.
Foster, C.V.L., R.C. Harris and D.H. Snow. 1988. The effect of oral L-carnitine supplementation on the muscle and plasma concentrations in the Thoroughbred horse. Comp. Biochem. Physiol. 91A(4):827-835.
Gannon, J.R. and R.V. Kendall. 1982. A clinical evaluation of N,N-dimethylglycine (DMG) and diisopropylammonium dichloroacetate (DIPA) on the performance of racing greyhounds. Canine Pract. 9(6):7-13.
Glade, M.J. 1991. Timed administration of leucine, isoleucine, and valine, glutamine, and carnitine to enhance athletic performance. Equine Athlete 4(5):4-10.
Hancock, D. 1992. Critical reading of the scientific literature. Bovine Proc. 24:29-38.
Hanson, R.R., L.R. Smalley, G.K. Huff, S. White and T.A. Hammad. 1997. Oral treatment with a glucosamine-chondroitin sulfate compound for degenerative joint disease in horses: 25 cases. Equine Pract. 19(9):16-22.
Harris, R.C., K. Sodehund and E. Hultman. 1992. Evaluation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 83:367-374.
Harris, R.C., M. Viru, P.L. Greenhaff and E. Hultman. 1993. The effect of oral creatine supplementation on running performance during short-term exercise in man. J. Physiol. (Lond.) 467:74P.
Iannuzzi, J. 1994. Pollen for horses? Amer. Bee J. 134:39-40.
Jones, W.E. 1997. Nutraceuticals for equine practice. J. Equine Vet. Sci. 17(11):562-572.
Lang, K.J., B.D. Nielsen, K.L. Waite, J. Link, G.M. Hill and M.W. Orth. 2001a. Increased plasma silicon concentrations and altered bone resorption in response to sodium zeolite A supplementation in yearling horses. J. Equine Vet. Sci. 21(11):550- 555.
Lang, K.J., B.D. Nielsen, K.L. Waite, G.M. Hill and M.W. Orth. 2001b. Supplemental silicon increases plasma and milk silicon concentrations in horses. J. Anim. Sci. 79:2627-2633.
Leffler, C.T., A.F. Philippi, S.G. Leffler, J.C. Mosure and P.D. Kim. 1999. Glucosamine, chondroitin, and manganese ascorbate for degenerative joint disease of the knee or low back: a randomized, double-blind, placebo-controlled pilot study. Military Med. 164(2)85-91.
Levine, S.B., G.D. Myhre, G.L. Smith, J.G. Burns and H. Erb. 1982. Effect of a nutritional supplement containing N, N-dimethylglycine (DMG) on the racing Standardbred. Equine Pract. 4(3):17-20.
Liebelt, R.A. and D. Calcagnetti. 1999. Effects of a bee pollen diet on the growth of the laboratory rat. Pp. 139:39.
Madsen, K., D.A. Maclean, B. Kiens and D. Christensen. 1996. Effects of glucose, glucose plus branched-chain amino acids, or placebo on bike performance over 100 km. J. Appl. Physiol. 81:2644-2650.
McCarthy, M.F. 1989. The neglect of glucosamine for the treatment of osteoarthritis-a personal perspective. Med. Hypotheses. 42(5):323-327.
Mello, D.M., B.D. Nielsen, T.L. Peters and M.W. Orth. 2001. Preliminary studies on the comparative effects of hexosamines in the inhibition of equine articular cartilage degradation. Proc. Equine Nutr. Phys. Symp. Pp. 55-60.
Metcalf, J. 1983. MSM – a dietary derivative of DMSO. J. Equine Vet. Sci. 3:148, 174-175.
National Research Council. 1989. Nutrient Requirements of Horses. 5th ed., National Academy Press, Washington, DC.
Nerucci, F., A. Fioravanti, M.R. Cicero, G. Collodel and R. Marcolongo. 2000. Effects of chondroitin sulfate and interleukin-B on human chondrocyte cultures exposed to pressurization: a biochemical and morphological study. Osteoarthritis Cart. 8:279-287.
Nielsen, F.H. 1991. Nutritional requirements for boron, silicon, vandium, nickel, and arsenic: current knowledge and speculation. FASEB J. 5:2661- 2667.
Nielsen, B.D., G.D. Potter, E.L. Morris, T.W. Odom, D.M. Senor, J.A. Reynolds, W.B. Smith, M.T. Martin and E.H. Bird. 1993. Training distance to failure in young racing Quarter Horses fed sodium zeolite. A. J. Equine Vet. Sci. 13(10):562.
Nielsen, B.D., J.I. Fenton, K.A. Chlebek-Brown, C.D. Corn, K.S. Waite, M.W. Orth and J.P. Caron. 1998. Longeing and glucosamine supplementation on known biological markers of bone and joint metabolism. 17th Assoc. Equine Sports Med. Mar 5-8, Leesburg, VI. Pp. 41-45.
Piperno, M., P. Reboul, M.P. Hellio Le Graverand, M.J. Peschard, M. Annefeld, M. Richard and E Vignon. 2000. Glucosamine sulfate modulates dysregulated activities of human osteoarthritic chondrocytes in vitro. Osteoarthr. Cart. 8:207- 212.
Reeves, P.G. 1997. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 127:838S-841S.
Rose, R.J., H.A. Schlierf, P.K. Knight, C. Plummer, M. Davis and S.P. Ray. 1989. Effects of N,Ndimethylglycine on cardiorespiratory function and lactate production in Thoroughbred horses performing incremental treadmill exercise. Vet. Rec. 125:268-271.
Sandy, J.D., D.G. Gamett, V. Thompson and C. Verscharen. 1998. Chondrocyte-mediated catabolism of aggrecan: aggrecanase-dependent cleavage induced by interleukin-1 or retinoic acid can be inhibited by glucosamine. Biochem. J. 335:59-66.
Setnikar, I., C. Giacchetti and G. Zanolo. 1986. Pharmokinetics of glucosamine in the dog and in man. Drug Res. 36(I):729-735.
Snow, D.H. 1992. A review of nutritional aids to energy production for athletic performance. The Equine Athlete. 5(5):1-10.
Speedhorse. 2001. Commission makes no changes to All American field. Speedhorse 25(35):8.
Trottier, N.L., B.D. Nielsen, K.J. Lang, P.K. Ku and H.C. Schott. 2002. Endurance exercise alters serum branched-chain amino acids and alanine concentrations. Accepted Equine Vet. J.
Uebelhart, D., E.J.-M.A. Thonar, P.D. Delmas, A. Chantraine and E. Vignon. 1998. Effects of oral chondroitin sulfate on the progression of knee osteoarthritis: a pilot study. Osteo. Cart. 6(Supp. A):39-46.
Warren, L.K., L.M. Lawrence and K.N. Thompson. 1999. The influence of betaine on untrained and trained horses exercising to fatigue. J. Anim. Sci. 77:677-684.
Williams, M.H. 1992. Ergogenic and ergolytic substances. Med. Sci. Sports Exercise 24:S344- S348.
Other confounding variables may also have played a role in the improved performance. Well-controlled studies in humans have not shown consistent ergogenic effects (Bucci, 1993); and in a crossover study using a placebo and DMG, Rose et al. (1989) found no beneficial effects on cardiovascular function or lactate accumulation in the exercising horse. One study finding beneficial effects of DMG administration in horses was done by Levine et al. (1982). Comparing horses given DMG to those not receiving any, blood samples were taken after exercising on six separate days for the treated horses but only on four days for the control horses.
Though treated horses were reported to have lower blood lactic acid concentrations after exercising than the controls, it is interesting to note that DMG did not decrease the lactate concentrations from those recorded at the beginning of the study. If DMG was truly efficacious in lowering blood lactate concentrations, one might expect lower concentrations after horses had been consuming the product for a period of time. This discrepancy was never explained. Also in this study, a major point was that trainers subjectively claimed treated horses were “more aggressive”, had “better appetites and attitudes”, and were able “to recover faster from racing and training than the controls”. As a critical reviewer of a study, one needs to question whether this was just a perceived effect, as the trainers were not blinded to the treatments. All too often if a trainer believes a product will improve the attitude of a horse, that trainer will testify that such a change has occurred.
Conclusions
The nutraceuticals discussed in this review are just a few of those available on the market. Though it would be nice if one could decide conclusively whether a given supplement is efficacious, it is extremely difficult to do so. As can be seen, even if a product appears to work in a given study, if the research design is flawed, the evidence must be viewed with skepticism. Alternatively, if a properly controlled study fails to show an advantage in treated animals compared to control animals, it still does not prove the product did not work if a sufficient number of research subjects was not used. With the possibility of huge sums of money to be won by having only a very small improvement in performance, it is reasonable to expect horse owners and trainers will continue to use nutraceuticals whether their efficacy has been proven or not.
References
Adebowale, A.O., D.S. Cox, Z. Liang and N.D. Eddington. 2000. Analysis of glucosamine and chondroitin sulfate content in marketed products and the Caco-2 permeability of chondroitin sulfate raw materials. J. Amer. Nutraceutical Assoc. 3(1):37-44.
Algner, T., S.I. Vornehm, G. Zeidler, J. Dudhia, K. Von der Mark and M.T. Bayliss. 1997. Suppression of cartilage matrix gene expression in upper zone chondrocytes of osteoarthritic cartilage. Arthritis Rheum. 40:562-569.
Bassleer, C., Y. Henrotin and P. Franchimont. 1992. In vitro evaluation of drugs proposed as chondroprotective agents. Int. J. Tiss. Reac. 14(5):231-241.
Boothe, D.M. 1997. Nutraceuticals in veterinary medicine Part 1: Definitions and regulations. Comp. Cont. Ed. 19(11).
Bremer, J. 1983. Carnitine-metabolism and functions. Physiol. Rev. 63:1420-1480.
Bucci, L. 1993. Nutrients as ergonic aids for sports and exercise. Boca Raton, CRC Press.
Calders, P., J.L. Pannier, D.M. Matthys and E.M. Lacroix. 1997. Pre-exercise branched-chain amino acid administration increases endurance performance in rats. Med. Sci. Sports Exerc. 29(9):1182-1186.
Carlisle, E.M. 1972. Silicon: An essential element for the chick. Science, Pp. 178:619.
Casini, L., D. Gatta, L. Magni and B. Colombani. 2000. Effect of prolonged branched-chain amino acid supplementation on metabolic response to anaerobic exercise in Standardbreds. J. Equine Vet. Sci. 20:120-123.
Etchebarne, M. 2002. Dairy nutrition in the west. Nutrition Seminar. Michigan State University. Feb. 11.
FDA. 1995. FDA’s view of dietary supplement legislation. FDA Vet. May/June.
Foster, C.V.L. and R.C. Harris. 1989. Plasma carnitine concentrations in the horse following oral supplementation using a triple dose regime. Equine Vet. J. 21:376-377.
Foster, C.V.L., R.C. Harris and D.H. Snow. 1988. The effect of oral L-carnitine supplementation on the muscle and plasma concentrations in the Thoroughbred horse. Comp. Biochem. Physiol. 91A(4):827-835.
Gannon, J.R. and R.V. Kendall. 1982. A clinical evaluation of N,N-dimethylglycine (DMG) and diisopropylammonium dichloroacetate (DIPA) on the performance of racing greyhounds. Canine Pract. 9(6):7-13.
Glade, M.J. 1991. Timed administration of leucine, isoleucine, and valine, glutamine, and carnitine to enhance athletic performance. Equine Athlete 4(5):4-10.
Hancock, D. 1992. Critical reading of the scientific literature. Bovine Proc. 24:29-38.
Hanson, R.R., L.R. Smalley, G.K. Huff, S. White and T.A. Hammad. 1997. Oral treatment with a glucosamine-chondroitin sulfate compound for degenerative joint disease in horses: 25 cases. Equine Pract. 19(9):16-22.
Harris, R.C., K. Sodehund and E. Hultman. 1992. Evaluation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 83:367-374.
Harris, R.C., M. Viru, P.L. Greenhaff and E. Hultman. 1993. The effect of oral creatine supplementation on running performance during short-term exercise in man. J. Physiol. (Lond.) 467:74P.
Iannuzzi, J. 1994. Pollen for horses? Amer. Bee J. 134:39-40.
Jones, W.E. 1997. Nutraceuticals for equine practice. J. Equine Vet. Sci. 17(11):562-572.
Lang, K.J., B.D. Nielsen, K.L. Waite, J. Link, G.M. Hill and M.W. Orth. 2001a. Increased plasma silicon concentrations and altered bone resorption in response to sodium zeolite A supplementation in yearling horses. J. Equine Vet. Sci. 21(11):550- 555.
Lang, K.J., B.D. Nielsen, K.L. Waite, G.M. Hill and M.W. Orth. 2001b. Supplemental silicon increases plasma and milk silicon concentrations in horses. J. Anim. Sci. 79:2627-2633.
Leffler, C.T., A.F. Philippi, S.G. Leffler, J.C. Mosure and P.D. Kim. 1999. Glucosamine, chondroitin, and manganese ascorbate for degenerative joint disease of the knee or low back: a randomized, double-blind, placebo-controlled pilot study. Military Med. 164(2)85-91.
Levine, S.B., G.D. Myhre, G.L. Smith, J.G. Burns and H. Erb. 1982. Effect of a nutritional supplement containing N, N-dimethylglycine (DMG) on the racing Standardbred. Equine Pract. 4(3):17-20.
Liebelt, R.A. and D. Calcagnetti. 1999. Effects of a bee pollen diet on the growth of the laboratory rat. Pp. 139:39.
Madsen, K., D.A. Maclean, B. Kiens and D. Christensen. 1996. Effects of glucose, glucose plus branched-chain amino acids, or placebo on bike performance over 100 km. J. Appl. Physiol. 81:2644-2650.
McCarthy, M.F. 1989. The neglect of glucosamine for the treatment of osteoarthritis-a personal perspective. Med. Hypotheses. 42(5):323-327.
Mello, D.M., B.D. Nielsen, T.L. Peters and M.W. Orth. 2001. Preliminary studies on the comparative effects of hexosamines in the inhibition of equine articular cartilage degradation. Proc. Equine Nutr. Phys. Symp. Pp. 55-60.
Metcalf, J. 1983. MSM – a dietary derivative of DMSO. J. Equine Vet. Sci. 3:148, 174-175.
National Research Council. 1989. Nutrient Requirements of Horses. 5th ed., National Academy Press, Washington, DC.
Nerucci, F., A. Fioravanti, M.R. Cicero, G. Collodel and R. Marcolongo. 2000. Effects of chondroitin sulfate and interleukin-B on human chondrocyte cultures exposed to pressurization: a biochemical and morphological study. Osteoarthritis Cart. 8:279-287.
Nielsen, F.H. 1991. Nutritional requirements for boron, silicon, vandium, nickel, and arsenic: current knowledge and speculation. FASEB J. 5:2661- 2667.
Nielsen, B.D., G.D. Potter, E.L. Morris, T.W. Odom, D.M. Senor, J.A. Reynolds, W.B. Smith, M.T. Martin and E.H. Bird. 1993. Training distance to failure in young racing Quarter Horses fed sodium zeolite. A. J. Equine Vet. Sci. 13(10):562.
Nielsen, B.D., J.I. Fenton, K.A. Chlebek-Brown, C.D. Corn, K.S. Waite, M.W. Orth and J.P. Caron. 1998. Longeing and glucosamine supplementation on known biological markers of bone and joint metabolism. 17th Assoc. Equine Sports Med. Mar 5-8, Leesburg, VI. Pp. 41-45.
Piperno, M., P. Reboul, M.P. Hellio Le Graverand, M.J. Peschard, M. Annefeld, M. Richard and E Vignon. 2000. Glucosamine sulfate modulates dysregulated activities of human osteoarthritic chondrocytes in vitro. Osteoarthr. Cart. 8:207- 212.
Reeves, P.G. 1997. Components of the AIN-93 diets as improvements in the AIN-76A diet. J. Nutr. 127:838S-841S.
Rose, R.J., H.A. Schlierf, P.K. Knight, C. Plummer, M. Davis and S.P. Ray. 1989. Effects of N,Ndimethylglycine on cardiorespiratory function and lactate production in Thoroughbred horses performing incremental treadmill exercise. Vet. Rec. 125:268-271.
Sandy, J.D., D.G. Gamett, V. Thompson and C. Verscharen. 1998. Chondrocyte-mediated catabolism of aggrecan: aggrecanase-dependent cleavage induced by interleukin-1 or retinoic acid can be inhibited by glucosamine. Biochem. J. 335:59-66.
Setnikar, I., C. Giacchetti and G. Zanolo. 1986. Pharmokinetics of glucosamine in the dog and in man. Drug Res. 36(I):729-735.
Snow, D.H. 1992. A review of nutritional aids to energy production for athletic performance. The Equine Athlete. 5(5):1-10.
Speedhorse. 2001. Commission makes no changes to All American field. Speedhorse 25(35):8.
Trottier, N.L., B.D. Nielsen, K.J. Lang, P.K. Ku and H.C. Schott. 2002. Endurance exercise alters serum branched-chain amino acids and alanine concentrations. Accepted Equine Vet. J.
Uebelhart, D., E.J.-M.A. Thonar, P.D. Delmas, A. Chantraine and E. Vignon. 1998. Effects of oral chondroitin sulfate on the progression of knee osteoarthritis: a pilot study. Osteo. Cart. 6(Supp. A):39-46.
Warren, L.K., L.M. Lawrence and K.N. Thompson. 1999. The influence of betaine on untrained and trained horses exercising to fatigue. J. Anim. Sci. 77:677-684.
Williams, M.H. 1992. Ergogenic and ergolytic substances. Med. Sci. Sports Exercise 24:S344- S348.
Related topics:
Recommend
Comment
Share

Would you like to discuss another topic? Create a new post to engage with experts in the community.