Integration of nutrition and health: implications for equine performance
Published: February 19, 2007
By: JOHN R. KOHNKE - John Kohnke Consultancy Pty Ltd. (Courtesy of Alltech Inc.)
It is well established that horses require an adequate and balanced diet to grow, reproduce and exercise for a wide range of purposes. The affluent lifestyle in developed countries has increased the popularity of horses for racing, equestrian and leisure activities. This has resulted in horses being kept on smaller grazing areas in the urban fringe areas of cities or boarded in training stables and barns of equestrian centers. A more intensive form of management has developed with the need to provide stored feeds including grains, hays and haylage, with a reduced reliance on fresh pasture as a basis for horse diets. As an example, the volume of grain, hay and prepared feeds destined for horses has doubled over the last decade in Australia (Kohnke et al., 1999). The use of stored feeds has been a necessity to provide for horses over-wintering in stables and barns in cold northern hemisphere countries or during drought periods on hot, dry continents.
The increasing reliance on processed grains and commercially prepared feeds formulated specifically to meet the nutritional needs of racing and upper level equestrian event horses has resulted in the availability of a wide variety of mixed feeds fortified with minerals, trace minerals and vitamins. Separate nutritional supplements fed to correct known or perceived inadequate or imbalanced levels or a low dietary content of these important nutrients are also popular additives to horse feeds. Indeed, one of the most common queries raised by horse owners and trainers is related to the need for, and the benefits of, dietary supplements to improve health, avoid marginal deficiencies and to ensure the optimum performance and appearance of competitive horses. Owners want their horses to look and perform well, and often no cost is spared in supplementing the diet to achieve ultimate success in competition.
Natural nutrient content of feeds
The widespread analysis of the nutrient composition of common feeds offered to horses has identified seasonal and locality variations that can result in marginal, low or inadequate levels of trace minerals. Trace mineral content can be influenced by soil pH, leaching and calcium and phosphorus content to a greater degree than other macro- and micronutrients (Kohnke et al., 1999). Each feed ingredient has a characteristic nutrient content, which can vary with variety and stage of growth and harvest, as well as with soil pH and fertility, fertilizer application, rainfall and seasonal growing conditions. The stage of growth ay harvest, duration and conditions of storage and final processing prior to feeding can all influence the vitamin content, especially when these processes involve heat and oxidation stress on sensitive vitamin precursors (carotenes, sterols, tocopherols) and active vitamins (BASF, 1994).
There is also a net shift of nutrients through the pasture micro environment, with up to 80% of the phosphorus, trace minerals and electrolytes such as potassium eliminated in faeces and subsequently recycled through the pasture (Kelleher, 1995). However the greatest net increase in concentration of trace minerals in grazing horses occurs within the ‘roughs’ or defecation areas, which can account for up to 52% of the total pasture area. (Archer, 1973; Odberg and Francis-Smith, 1977). The concentration of faecal and urinary wastes in these areas can result in a transfer of trace elements from established grazing areas to the roughs over time. This in turn, can increase the risk of dietary deficiencies of these nutrients in horses maintained predominately on pasture for prolonged periods without supplementation. (Kelleher, 1995; Goold, 1991).
The time constraints and the costs of analyzing batches of feedstuffs other than for macronutrients such as energy, crude protein, crude fat and fibre content, including the major minerals calcium and phosphorus in some cases, has resulted in feed manufacturers adding a generalized range of minerals, trace minerals, electrolytes, vitamins and digestive aids to fortify commercially blended feeds. Industry practices for nutrient addition range from 25-100% of NRC (1989) guidelines for specific classes of horses.
Over the last decade, the NRC (1989) guidelines for horses have been reviewed by a number of authors (Hintz, 2000) to establish more up-to-date guidelines for nutrient needs and hence supplementation. While there is arguably a need for additional supplementation of the majority of diets fed to racing and performance horses, it is the base mix, form and chemical stability of trace mineral and vitamins used to compound these fortified feeds and supplements that is discussed in this paper. Nutrient interactions and incompatibilities If supplementary minerals, trace minerals and vitamins are used to fortify feeds to meet nutritional demand, it is prudent to ensure that optimum bioavailability and bioactivity is assured. Scientifically formulated commercially prepared dry and moist molasses and fat added to sweet and high energy feeds have become increasingly popular because of the savings in preparation and feed-out time, improved palatability and acceptance and the assumption that these feeds or nutritional supplements contain balanced and adequate levels of major nutrients to meet the needs of specific classes of horses. However, in some cases, inherent chemical interactions and nutrient incompatibilities within the premix can occur. These interactions combined with the degrading effects of processing, moisture content and extended storage times of the mixed feeds, can reduce or significantly affect the bioavailability, activity and benefit from individual minerals, trace minerals and vitamins added to these feeds.
A number of reviews have been published detailing the factors that influence the degradation of vitamins in feeds. These include inter-vitamin incompatibilities, chemical and physical ‘stresses’ in premixes and the form of processing used to compound feeds for horses and other livestock (Gadient, 1986; Coelho, 1991). The multinational vitamin manufacturers are able to provide stability data and recommendations on the stability characteristics and shelf life of natural and synthetic vitamins (BASF, 1994). Moreover, recognized chemical manuals (eg., Merck, 1996) and pharmacology references (Martindale, 1999; USP, 1999) provide comprehensive details of nutrient and drug interactions and chemical incompatibilities as well as storage recommendations. These guidelines can be used as a basis for compounding premixes and blends to ensure maximum stability, bioavailability, utilization and health benefit to the recipient animal.
Unfortunately, many of these established incompatibilities and degradation influences are ignored by both human and veterinary supplement formulators. Cost constraints in the competitive generic supplement and livestock feed industry often limit innovation in formulation, use of appropriate types and concentration of antioxidants, stabilizers and bioplexed trace minerals and suitable packaging to help maintain optimum stability during storage of premixes and compounded feeds . There are a number of important formulation guidelines that should be observed when compounding feeds and supplements that contain minerals, trace minerals and vitamins.
INFLUENCE OF BASE MIX FORMULATION
The base or carriers for a trace mineral and vitamin supplement are usually the cheapest of the feedgrade chemical powders that are readily available. These include mildly alkaline carriers such as microfined limestone, magnesium oxide or potassium chloride. The alkalinity of these base mixes can reduce the bioavailability and accelerate the degradation rate of vitamins in premixes. The majority of vitamins are more stable in slightly acidic conditions, with pH values of 4-6 in semi-moist feeds or liquid supplements, in particular (Table 2). For example, it has been established that high concentrations of alkaline calcium carbonate, as microfined limestone used as a carrier in supplements, or as a major component of the fines in mixed feeds, can reduce the bioavailability of a number of trace minerals such as iron, magnesium and zinc (Lewis, 1995). As a result, overages must be established by stability testing over the proposed expiry time in order to offset known binding effects or losses (BASF, 1994).
Table 1. Effect of trace mineral source on vitamin retention.
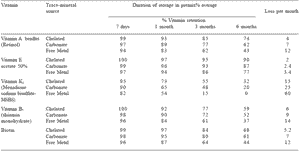
To enlarge the image, click here
Adapted from BASF (1994).
Less reactive and binding carriers include ground linseed meal, milk powder, whey powder, wheat or cereal grain offal and wheat or rice flour and fine middlings.
Major advances in protecting trace minerals by chelation and protein bioplex combinations help reduce interaction and improve the uptake and utilization of the trace minerals. Vitamins can be protected against trace mineral interaction and oxidation by gelatin coatings, ethyl cellulose and silica films and cross linked polymer coated beads to increase the shelf life time of vitamin mixtures in dry powder, pelleted or semi-moist feeds (BASF, 1994). The development of more stable forms of vitamins has helped in reducing the binding and chemical degradation of micronutrients such as trace minerals and ‘stress’ factors on vitamins. As an example, use of chelated or bioplexed trace minerals in premixes, which are incapable of forming destructive free radicals, can directly improve the stability of a number of key vitamins in premixes, as summarized in Table 1 (BASF, 1994).
INFLUENCE OF PROCESSING
The manufacturers of milling and processing machinery such as pelleters, expanders, extruders and a combination of these machines are well aware of the effects of heat and pressure-related processes on the stability of vitamins. Often they can provide guidelines on vitamin losses related to manufacturing variables and time. These data can be used to establish the overage required to offset known losses during the manufacturing process to ensure adequate nutrient activity is maintained during the storage time to expiry of vitamin premixes used in blended feeds.
‘STRESS’ FACTORS ON VITAMINS
It is well documented that vitamins are sensitive to a number of stress factors (BASF, 1994; Martindale 1999). This can affect the native or natural content of vitamin precursors and active vitamins in feed ingredients from the time of harvest to the feed bin. These destructive actions reduce the bioactivity over time of natural or synthetic vitamins added to fortify premixes, supplements and even nutritionally based or enhanced medicinal products.
These factors include physical stresses to which the individual vitamin is subjected during processing, manufacture and storage prior to ingestion by the recipient animal. Physical stresses may include exposure to ultraviolet light (sunlight), heat and oxidation (by aeration), pressure (and heat) via pelleting, tableting and extrusion, and exposure to moisture (particularly with heat and aeration) during operations to improve the digestibility of starch and protein or reduce wastage and selection. Such operations include consumption extrusion, micronizing, expanding and steam pelleting.
Other external destructive effects include chemical interaction with iron, copper, zinc and heavy metals, which can destroy vitamin activity to varying degrees (Table 2). Internal chemical interactions tend, however, to be overlooked as a cause of vitamin destruction in premixes and especially in powder, paste and liquid feed supplements (Kohnke, 1986). The risk of chemical destruction is relative to the concentration and degree of dissociation of certain chemicals used in the formulation (BASF, 1994).
Chemical stresses may include direct contact between incompatible vitamins facilitated by moisture, presence of oxidising agents, heavy metal impurities and unprotected trace minerals and incorrect pH range. The grade or degree of purity and formulation of the vitamin carriers also influences the likely risk of, and rate of, destruction.
Table 2. Factors affecting stability of vitamins.
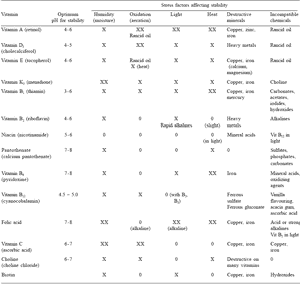
To enlarge the image, click here
O - not sensitive X – sensitive XX – very sensitive
(Adapted from Gadient, 1986; Kohnke, 1986; BASF, 1994)
Pharmaceutical or technical grade vitamins to USP and BP standards are considered more stable as potentially destructive impurities are removed (BASF, 1994). Certain radicals and stabilizers are incorporated to protect the vitamins against interaction, oxidation or hygroscopic damage. Feed grade vitamins tend to be less purified or stabilized (BASF, 1994). However, they have a lower inclusion cost in price-competitive feeds for a large scale livestock production. Usually horse and small animal supplements command a higher price per unit of nutrient than supplement premixes for intensive livestock industries. This is influenced by small production runs and the assumption that most horse owners are affluent and can afford more costly packaging with a resultant higher net return throughout the marketing and distribution chain.
Recommendations for compounding mineral, trace mineral and vitamin premixes and feed supplements There are a number of important guidelines to consider when formulating feeds and supplements for horses and other animals. Careful formulation to reduce binding, incompatible chemical and known nutrient interactions will help reduce the need for excessive overages and thus reduce formulation costs, as well as extend the shelf life, and finally the bioavailability, utilization and health benefit to the recipient animal.
CHOOSE COMPATIBLE BASE MIXES AND CARRIERS
The bioavailability and utilization of trace minerals can be optimized by incorporating organic base mix carriers such as cereal grain and oilseed meals rather than mineral compounds such as calcium and magnesium carbonates, oxides and excess amounts of alkalinizing sodium bicarbonate, in particular. The stability of vitamins can be adversely affected by alkaline carriers such as carbonates, bicarbonates, sulfates and oxides. Unprotected trace mineral contaminates including copper, zinc and iron within the carrier can also adversely affect the stability (BASF, 1994).
INCORPORATE CHELATED OR BIOPLEXED TRACE MINERALS
Where possible, amino acid chelated or bioplexed trace minerals should be used at the highest practical levels to optimize bioavailability of the individual trace mineral, as well as to reduce the degradation effect inorganic mineral forms have on many vitamins by shielding them against oxidative effects of free radicals (BASF, 1994).
COMPOUND WITH COATED VITAMINS
Major vitamin manufacturers can provide recommendations on the most stable form of coated vitamins relative to the base mix and trace mineral type and inclusion rate in a premix or compounded feed. The fat soluble vitamins (vitamin A, vitamin D3, vitamin E and vitamin K3) are most affected by oxidation; and high fat inclusion rates to boost energy and inorganic trace minerals exacerbate this process (BASF, 1994). Fat soluble vitamin loss can be minimized by inclusion of antioxidants and amino acid chelated (bioplexed) trace minerals.
ADEQUATE LEVELS OF ANTIOXIDANTS
The fat soluble vitamins as well as vitamin B1, vitamin B2, vitamin B12, vitamin C and choline are all affected by oxidation due to aeration during processing, packing and storage. Manufacturers supply the pure vitamins in vacuum packed or nitrogen sealed containers to minimize oxidation during storage prior to blending. However, once incorporated into premixes, compounded feeds, the oxidative destruction during storage can result in significant deterioration and loss of bioactivity of the vitamin.
Manufacturers of antioxidant compounds can provide recommendations regarding the type and concentration of antioxidants that will provide optimum protection of vitamins in dry powder, semimoist and liquid formulations.
AVOID VITAMIN INCOMPATIBILITIES
As summarized in Table 2, vitamins are prone to a number of stress factors that accelerate their destruction in premixes and compound feeds. However, incompatibilities exist between individual vitamins when in contact within semi-moist or liquid formulations (Kohnke, 1986). For example, vitamin B1 is incompatible with vitamin B2 and vitamin B12 (Table 2). Choline chloride is destructive to other B vitamins when mixed in feeds or supplements, although it too deteriorates during storage (Tables 1 and 2). In fact, if possible, choline chloride should be included in mineral or trace mineral premixes rather than in vitamin premixes to reduce its potentially destructive effect.
However, in dry powder premixes and compounded feeds, the risk can be minimized by using coated vitamins and less concentrated formulations. Thiamin nitrate is less affected by choline/trace mineral premixes under acidic high moisture conditions than thiamin monohydrate (BASF, 1994). The inclusion of strong alkalinizing compounds, especially bicarbonates, should be minimized or avoided in mixes containing B vitamins, which are particularly sensitive to an alkaline environment during storage.
Conclusion
The increasing reliance on processed grains and commercially prepared feeds formulated specifically to meet the nutritional needs of racing and upper level equestrian event horses has resulted in the availability of a wide variety of mixed feeds fortified with minerals, trace minerals and vitamins. Separate nutritional supplements fed to correct known or perceived inadequate or imbalanced levels or a low dietary content of these important nutrients are also popular additives to horse feeds. Indeed, one of the most common queries raised by horse owners and trainers is related to the need for, and the benefits of, dietary supplements to improve health, avoid marginal deficiencies and to ensure the optimum performance and appearance of competitive horses. Owners want their horses to look and perform well, and often no cost is spared in supplementing the diet to achieve ultimate success in competition.
Natural nutrient content of feeds
The widespread analysis of the nutrient composition of common feeds offered to horses has identified seasonal and locality variations that can result in marginal, low or inadequate levels of trace minerals. Trace mineral content can be influenced by soil pH, leaching and calcium and phosphorus content to a greater degree than other macro- and micronutrients (Kohnke et al., 1999). Each feed ingredient has a characteristic nutrient content, which can vary with variety and stage of growth and harvest, as well as with soil pH and fertility, fertilizer application, rainfall and seasonal growing conditions. The stage of growth ay harvest, duration and conditions of storage and final processing prior to feeding can all influence the vitamin content, especially when these processes involve heat and oxidation stress on sensitive vitamin precursors (carotenes, sterols, tocopherols) and active vitamins (BASF, 1994).
There is also a net shift of nutrients through the pasture micro environment, with up to 80% of the phosphorus, trace minerals and electrolytes such as potassium eliminated in faeces and subsequently recycled through the pasture (Kelleher, 1995). However the greatest net increase in concentration of trace minerals in grazing horses occurs within the ‘roughs’ or defecation areas, which can account for up to 52% of the total pasture area. (Archer, 1973; Odberg and Francis-Smith, 1977). The concentration of faecal and urinary wastes in these areas can result in a transfer of trace elements from established grazing areas to the roughs over time. This in turn, can increase the risk of dietary deficiencies of these nutrients in horses maintained predominately on pasture for prolonged periods without supplementation. (Kelleher, 1995; Goold, 1991).
The time constraints and the costs of analyzing batches of feedstuffs other than for macronutrients such as energy, crude protein, crude fat and fibre content, including the major minerals calcium and phosphorus in some cases, has resulted in feed manufacturers adding a generalized range of minerals, trace minerals, electrolytes, vitamins and digestive aids to fortify commercially blended feeds. Industry practices for nutrient addition range from 25-100% of NRC (1989) guidelines for specific classes of horses.
Over the last decade, the NRC (1989) guidelines for horses have been reviewed by a number of authors (Hintz, 2000) to establish more up-to-date guidelines for nutrient needs and hence supplementation. While there is arguably a need for additional supplementation of the majority of diets fed to racing and performance horses, it is the base mix, form and chemical stability of trace mineral and vitamins used to compound these fortified feeds and supplements that is discussed in this paper. Nutrient interactions and incompatibilities If supplementary minerals, trace minerals and vitamins are used to fortify feeds to meet nutritional demand, it is prudent to ensure that optimum bioavailability and bioactivity is assured. Scientifically formulated commercially prepared dry and moist molasses and fat added to sweet and high energy feeds have become increasingly popular because of the savings in preparation and feed-out time, improved palatability and acceptance and the assumption that these feeds or nutritional supplements contain balanced and adequate levels of major nutrients to meet the needs of specific classes of horses. However, in some cases, inherent chemical interactions and nutrient incompatibilities within the premix can occur. These interactions combined with the degrading effects of processing, moisture content and extended storage times of the mixed feeds, can reduce or significantly affect the bioavailability, activity and benefit from individual minerals, trace minerals and vitamins added to these feeds.
A number of reviews have been published detailing the factors that influence the degradation of vitamins in feeds. These include inter-vitamin incompatibilities, chemical and physical ‘stresses’ in premixes and the form of processing used to compound feeds for horses and other livestock (Gadient, 1986; Coelho, 1991). The multinational vitamin manufacturers are able to provide stability data and recommendations on the stability characteristics and shelf life of natural and synthetic vitamins (BASF, 1994). Moreover, recognized chemical manuals (eg., Merck, 1996) and pharmacology references (Martindale, 1999; USP, 1999) provide comprehensive details of nutrient and drug interactions and chemical incompatibilities as well as storage recommendations. These guidelines can be used as a basis for compounding premixes and blends to ensure maximum stability, bioavailability, utilization and health benefit to the recipient animal.
Unfortunately, many of these established incompatibilities and degradation influences are ignored by both human and veterinary supplement formulators. Cost constraints in the competitive generic supplement and livestock feed industry often limit innovation in formulation, use of appropriate types and concentration of antioxidants, stabilizers and bioplexed trace minerals and suitable packaging to help maintain optimum stability during storage of premixes and compounded feeds . There are a number of important formulation guidelines that should be observed when compounding feeds and supplements that contain minerals, trace minerals and vitamins.
INFLUENCE OF BASE MIX FORMULATION
The base or carriers for a trace mineral and vitamin supplement are usually the cheapest of the feedgrade chemical powders that are readily available. These include mildly alkaline carriers such as microfined limestone, magnesium oxide or potassium chloride. The alkalinity of these base mixes can reduce the bioavailability and accelerate the degradation rate of vitamins in premixes. The majority of vitamins are more stable in slightly acidic conditions, with pH values of 4-6 in semi-moist feeds or liquid supplements, in particular (Table 2). For example, it has been established that high concentrations of alkaline calcium carbonate, as microfined limestone used as a carrier in supplements, or as a major component of the fines in mixed feeds, can reduce the bioavailability of a number of trace minerals such as iron, magnesium and zinc (Lewis, 1995). As a result, overages must be established by stability testing over the proposed expiry time in order to offset known binding effects or losses (BASF, 1994).
Table 1. Effect of trace mineral source on vitamin retention.

To enlarge the image, click here
Adapted from BASF (1994).
Less reactive and binding carriers include ground linseed meal, milk powder, whey powder, wheat or cereal grain offal and wheat or rice flour and fine middlings.
Major advances in protecting trace minerals by chelation and protein bioplex combinations help reduce interaction and improve the uptake and utilization of the trace minerals. Vitamins can be protected against trace mineral interaction and oxidation by gelatin coatings, ethyl cellulose and silica films and cross linked polymer coated beads to increase the shelf life time of vitamin mixtures in dry powder, pelleted or semi-moist feeds (BASF, 1994). The development of more stable forms of vitamins has helped in reducing the binding and chemical degradation of micronutrients such as trace minerals and ‘stress’ factors on vitamins. As an example, use of chelated or bioplexed trace minerals in premixes, which are incapable of forming destructive free radicals, can directly improve the stability of a number of key vitamins in premixes, as summarized in Table 1 (BASF, 1994).
INFLUENCE OF PROCESSING
The manufacturers of milling and processing machinery such as pelleters, expanders, extruders and a combination of these machines are well aware of the effects of heat and pressure-related processes on the stability of vitamins. Often they can provide guidelines on vitamin losses related to manufacturing variables and time. These data can be used to establish the overage required to offset known losses during the manufacturing process to ensure adequate nutrient activity is maintained during the storage time to expiry of vitamin premixes used in blended feeds.
‘STRESS’ FACTORS ON VITAMINS
It is well documented that vitamins are sensitive to a number of stress factors (BASF, 1994; Martindale 1999). This can affect the native or natural content of vitamin precursors and active vitamins in feed ingredients from the time of harvest to the feed bin. These destructive actions reduce the bioactivity over time of natural or synthetic vitamins added to fortify premixes, supplements and even nutritionally based or enhanced medicinal products.
These factors include physical stresses to which the individual vitamin is subjected during processing, manufacture and storage prior to ingestion by the recipient animal. Physical stresses may include exposure to ultraviolet light (sunlight), heat and oxidation (by aeration), pressure (and heat) via pelleting, tableting and extrusion, and exposure to moisture (particularly with heat and aeration) during operations to improve the digestibility of starch and protein or reduce wastage and selection. Such operations include consumption extrusion, micronizing, expanding and steam pelleting.
Other external destructive effects include chemical interaction with iron, copper, zinc and heavy metals, which can destroy vitamin activity to varying degrees (Table 2). Internal chemical interactions tend, however, to be overlooked as a cause of vitamin destruction in premixes and especially in powder, paste and liquid feed supplements (Kohnke, 1986). The risk of chemical destruction is relative to the concentration and degree of dissociation of certain chemicals used in the formulation (BASF, 1994).
Chemical stresses may include direct contact between incompatible vitamins facilitated by moisture, presence of oxidising agents, heavy metal impurities and unprotected trace minerals and incorrect pH range. The grade or degree of purity and formulation of the vitamin carriers also influences the likely risk of, and rate of, destruction.
Table 2. Factors affecting stability of vitamins.

To enlarge the image, click here
O - not sensitive X – sensitive XX – very sensitive
(Adapted from Gadient, 1986; Kohnke, 1986; BASF, 1994)
Pharmaceutical or technical grade vitamins to USP and BP standards are considered more stable as potentially destructive impurities are removed (BASF, 1994). Certain radicals and stabilizers are incorporated to protect the vitamins against interaction, oxidation or hygroscopic damage. Feed grade vitamins tend to be less purified or stabilized (BASF, 1994). However, they have a lower inclusion cost in price-competitive feeds for a large scale livestock production. Usually horse and small animal supplements command a higher price per unit of nutrient than supplement premixes for intensive livestock industries. This is influenced by small production runs and the assumption that most horse owners are affluent and can afford more costly packaging with a resultant higher net return throughout the marketing and distribution chain.
Recommendations for compounding mineral, trace mineral and vitamin premixes and feed supplements There are a number of important guidelines to consider when formulating feeds and supplements for horses and other animals. Careful formulation to reduce binding, incompatible chemical and known nutrient interactions will help reduce the need for excessive overages and thus reduce formulation costs, as well as extend the shelf life, and finally the bioavailability, utilization and health benefit to the recipient animal.
CHOOSE COMPATIBLE BASE MIXES AND CARRIERS
The bioavailability and utilization of trace minerals can be optimized by incorporating organic base mix carriers such as cereal grain and oilseed meals rather than mineral compounds such as calcium and magnesium carbonates, oxides and excess amounts of alkalinizing sodium bicarbonate, in particular. The stability of vitamins can be adversely affected by alkaline carriers such as carbonates, bicarbonates, sulfates and oxides. Unprotected trace mineral contaminates including copper, zinc and iron within the carrier can also adversely affect the stability (BASF, 1994).
INCORPORATE CHELATED OR BIOPLEXED TRACE MINERALS
Where possible, amino acid chelated or bioplexed trace minerals should be used at the highest practical levels to optimize bioavailability of the individual trace mineral, as well as to reduce the degradation effect inorganic mineral forms have on many vitamins by shielding them against oxidative effects of free radicals (BASF, 1994).
COMPOUND WITH COATED VITAMINS
Major vitamin manufacturers can provide recommendations on the most stable form of coated vitamins relative to the base mix and trace mineral type and inclusion rate in a premix or compounded feed. The fat soluble vitamins (vitamin A, vitamin D3, vitamin E and vitamin K3) are most affected by oxidation; and high fat inclusion rates to boost energy and inorganic trace minerals exacerbate this process (BASF, 1994). Fat soluble vitamin loss can be minimized by inclusion of antioxidants and amino acid chelated (bioplexed) trace minerals.
ADEQUATE LEVELS OF ANTIOXIDANTS
The fat soluble vitamins as well as vitamin B1, vitamin B2, vitamin B12, vitamin C and choline are all affected by oxidation due to aeration during processing, packing and storage. Manufacturers supply the pure vitamins in vacuum packed or nitrogen sealed containers to minimize oxidation during storage prior to blending. However, once incorporated into premixes, compounded feeds, the oxidative destruction during storage can result in significant deterioration and loss of bioactivity of the vitamin.
Manufacturers of antioxidant compounds can provide recommendations regarding the type and concentration of antioxidants that will provide optimum protection of vitamins in dry powder, semimoist and liquid formulations.
AVOID VITAMIN INCOMPATIBILITIES
As summarized in Table 2, vitamins are prone to a number of stress factors that accelerate their destruction in premixes and compound feeds. However, incompatibilities exist between individual vitamins when in contact within semi-moist or liquid formulations (Kohnke, 1986). For example, vitamin B1 is incompatible with vitamin B2 and vitamin B12 (Table 2). Choline chloride is destructive to other B vitamins when mixed in feeds or supplements, although it too deteriorates during storage (Tables 1 and 2). In fact, if possible, choline chloride should be included in mineral or trace mineral premixes rather than in vitamin premixes to reduce its potentially destructive effect.
However, in dry powder premixes and compounded feeds, the risk can be minimized by using coated vitamins and less concentrated formulations. Thiamin nitrate is less affected by choline/trace mineral premixes under acidic high moisture conditions than thiamin monohydrate (BASF, 1994). The inclusion of strong alkalinizing compounds, especially bicarbonates, should be minimized or avoided in mixes containing B vitamins, which are particularly sensitive to an alkaline environment during storage.
Conclusion
An adequate and balanced diet including the presence of minerals, trace minerals and vitamins to correct low, imbalanced or inadequate natural levels in feeds or losses in vitamin activity due to processing and storage is paramount to the health and performance of horses. The effect of binding, chemical reaction and incompatibilities between nutrients should be considered when formulating premixes, compounded feeds and supplements.
Consideration in the selection of suitable base mix components and carriers within feeds and supplements and incorporation of amino acid chelated or bioplexed trace minerals to improve bioavailability and utilisation is important. As well, organic trace minerals reduce the risk of free mineral reaction, particularly of zinc, iron and especially copper, with a wide range of vitamins. The use of Bioplex trace minerals and coated vitamins will reduce the need for overages, minimise the rate of loss of bioactivity and increase the shelf life of the supplement or feed blend.
These actions, complemented by recommendations to minimize the physico-chemical stress factors on vitamins by limiting moisture and heat exposure during storage will result in a well formulated premix, feed or supplement that will maintain optimum bioavailability and bioactivity. In this way, a balanced and adequate diet can assist in improving the general health, productivity, ultimate soundness and performance of recipient animals.
References
- Archer, M. 1973. The species preferences of grazing horses. J Brit. Grassl. Soc. 38:128.
- BASF, 1994. Vitamin stability in premixes and feeds: A practical approach. Keeping Current. KC 9138 5th Revised Edition, BASF Fine Chemicals, USA.
- Coelho M. 1991. Fate of vitamins in premixes and feeds: vitamin stability. Feed Management 42:24. Cited in BASF Bulletin KC 9138.
- Gadient, M. 1986. Effect of pelleting on nutritional quality of feed. Maryland Nutrition Conference. Cited in BASF Bulletin KC9138. p. 93
- Goold. GT. 1991. The mineral content of Waikato thoroughbred horse pastures. The Major Elements. Proc. Equine Nutr. Sem., Nutrition Soc. of Aust. Canberra, Aust., pp. 199-124.
- Hintz H.F. 2000. Equine nutrition update. In: Proceedings. Am. Assoc. Equine. Pract. 46:62- 79.
- Kelleher, F.M. 1995. The ecology and management of horse pastures. Equine Nutritional and Pastures for Horses Workshop February 1995. Rural Industries Research and Development Corporation, Canberra, Aust.
- Kohnke, J.R. 1986. Liquid vitamin supplements: a guide to the evaluation. Equine Pract. 8(10):7-12.
- Kohnke J.R., F.M. Kelleher and P. Trevor-Jones. 1999. Feeding Horses in Australia. Publication No 99/49, Rural Industries Research and Development Corporation. Canberra, Aust, pp. 1, 129-130.
- Lewis, L.D. 1995. Minerals for horses. In: Equine Clinical Nutrition: Feeding and Care. Williams and Wilkins, Baltimore USA. 34:51-54.
- Martindale. 1999. The Complete Drug Reference. Vitamin monographs. The Pharmaceutical Press. London, UK.
- Merck. 1996. Vitamin monographs. The Merck Index. Merck and Co. Inc. Whitehouse Station N.J. USA.
- National Research Council. 1989. Nutrient Requirements of Horses. 5th edition. National Academy Press. Washington DC.
- Odberg, F.O and K. Frances-Smith. 1977. Studies on the formation of ungrazed eliminative areas in fields used by horses. Applied Animal Ethology. 3:27-34.
- United States Pharmacopoeia. 1999. Vitamin Monographs. United States Pharmacopoeal Convention. Inc. Rockdale, MD, USA.
Author: JOHN R. KOHNKE
John Kohnke Consultancy Pty Ltd, Rouse Hill, NSW, Australia
John Kohnke Consultancy Pty Ltd, Rouse Hill, NSW, Australia
Related topics
Join to be able to comment.
Once you join Engormix, you will be able to participate in all content and forums.
* Required information
Would you like to discuss another topic? Create a new post to engage with experts in the community.
Create a post